Definitions
Acid
Bronsted-Lowry
- An acid is a substance which donates a hydrogen ion to another substance
Lewis
- An acid is any compound that is a potential electron pair acceptor
Base
Bronsted-Lowry
- A base is a substance which accepts a hydrogen ion from another substance
Lewis
- A base is any compound that is a potential electron pair donor
pH
- pH = -log10 aH+
- Ref range 7.36 - 7.44
Buffer
- A buffer is a solution with the ability to minimise changes in pH when an acid or base is added to it
- A weak acid with its conjugate base
- Most effective at pH of pKa +/- 1 (in a closed system)
Henderson-Hasselbach Equation
- pH = pKa + log([HCO3-]/(0.03 x pCO2)) where pKa = 6.1 at 37C
Acidosis
- An abnormal process which would lower arterial pH if there were no secondary changes in response to the primary aetiological factor
Alkalosis
- An abnormal process which would raise arterial pH if there were no secondary changes in response to the primary aetiological factor
Acidaemia
- Excess acid in the blood
- arterial pH < 7.36
Alkalaemia
- Deficiency of acid in the blood
- arterial pH > 7.44
Simple Acid-Base Disorder
- There is a single, primary acid-base disorder
Mixed Acid-Base Disorder
- There are two or more primary acid-base disorders
Respiratory Acidosis
- An abnormal primary process in which paCO2 rises to a level higher than expected
Metabolic Acidosis
- An abnormal primary process that leads to an increase in fixed acids in the blood
Respiratory Alkalosis
- An abnormal primary process in which paCO2 falls to a level lower than expected
Metabolic alkalosis
- An abnormal primary process that causes [HCO3-] to rise to a level higher than expected
pH
Definition
- pH = -log10 aH+
- Ref range 7.36 - 7.44
Converting pH to [H+]
- pH 7.4 = [H+] 40nmol/L
- double [H+] → ↓ pH 0.3
| pH | [H+] |
|---|---|
| 7.36 | 44nmol/L |
| 7.4 | 40nmol/L |
| 7.44 | 36nmol/L |
The Importance of pH
- Effect on small molecules (Davis Hypothesis)
- Nearly every biosynthetic intermediate has at least one group that would be largely ionised at physiological pH, whether acid or base (pKa < 4.6 or > 9.2)
- eg phosphate, ammonium, carboxylic acid groups
- Exceptions: macromolecules, water insoluble lipids, waste products
- This effectively traps these ionised compounds within cells and organelles where they are required for their function
- Effect on large molecules
- Change pH → change net protein charge → change conformation → change function, ie altered enzyme function
alphastat vs pHstat
- Neutrality for an aqueous system is when [H+] = [OH-]
- pN = 0.5 x pKw (ion product of water)
- pKw is temp dependent
- Intracellular pH is maintained at around pN with changes in temp (pH 6.8 at 37C)
- For this to occur, require
- buffer with pKa ~ 0.5 x pKw
- present in sufficient concentration
- constant CO2 content
- Imidazole group of histidine residues is responsible for maintaining pH-temp relationship
- Imidazole groups have a fraction of ionization ( called alpha) of 0.55 in IC compartment which remains constant despite changes in temp (ie pK changes with temp)
- 1C ↓
temp →
- ↑ pH blood by 0.015
- ↓ paCO2 4.5% (due to ↑ solubility, CO2 content remains constant)
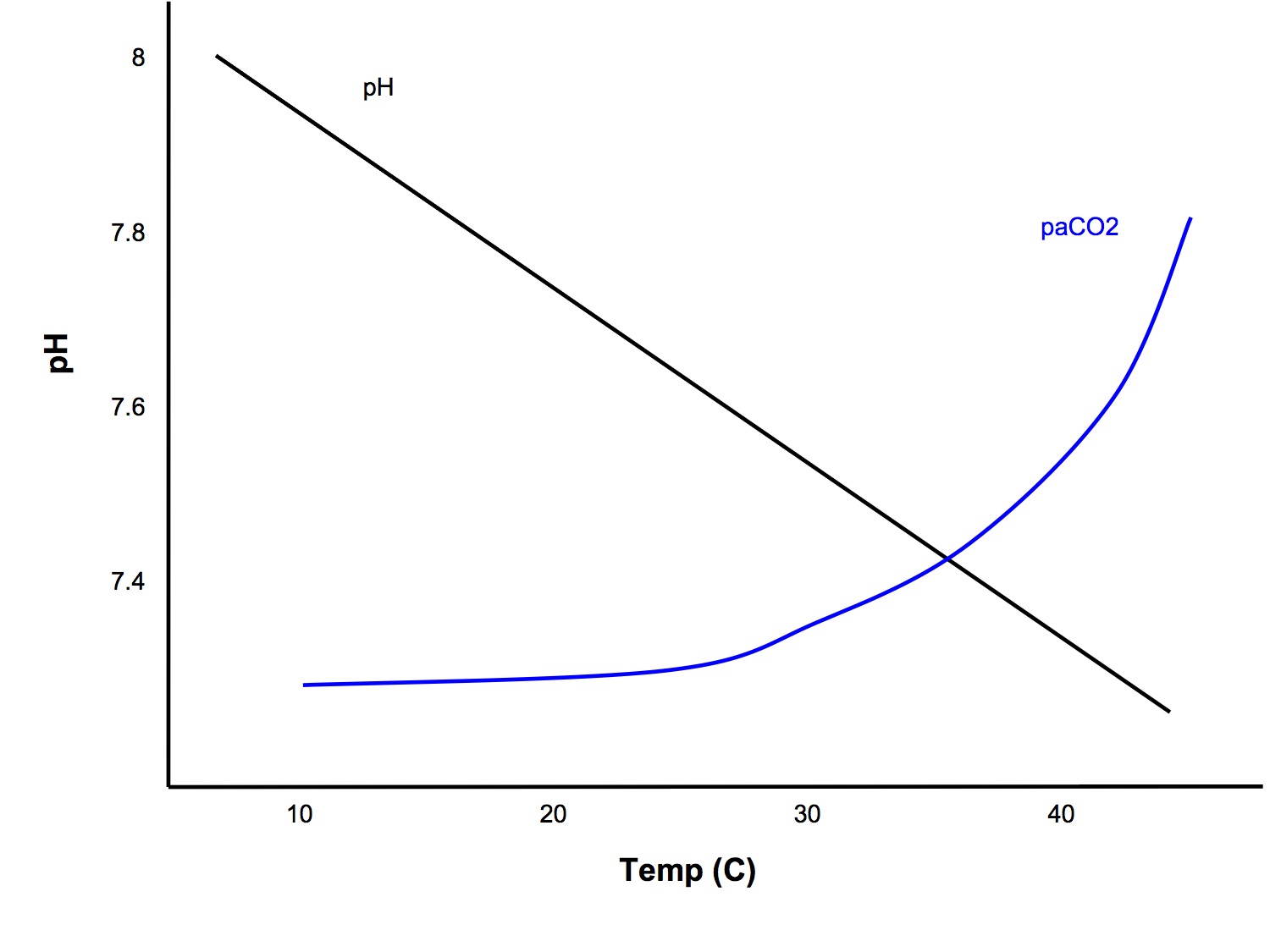
pH stat Hypothesis
- School of thought that blood pH should be maintained constant, ie at 7.4, despite changes in temp
- In this case, you should input the patient's temp into the ABG machine and interpret it using the values given for the patient's actual temperature
alphastat Hypothesis
- School of thought that one should aim to maintain constant net charge on all proteins (alpha) despite changes in temp
- In this case, you can leave the patient temp at the default of 37C when processing ABGs and interpret the results against the standard reference ranges
See Kerry Brandis for an excellent synopsis
Acid Production and Elimination
Respiratory Acids
- aka volatile acids
- 200ml/min CO2 produced by tissue metabolism
- 200ml/min → 200 x 60 x 24 L/day ÷ 22.4L/mol =
- 12, 857 mmol/day produced
- Eliminated by the lungs
- NB CO2 reacts with water to form carbonic acid. CO2 is not an acid according to the Bronsted-Lowry definition, but is according to the Lewis definition
- CO2 + H2O → H2CO3 → H+ + HCO3-
Metabolic Acids
- aka non-volatile/fixed acids
- Net production 1-1.5mmol/kg/day =
- 70 - 100 mmol/day produced
- Eliminated by the kidneys
- Produced due to incomplete metabolism of
- CHO → lactic acid
- fat → ketoacids
- protein → sulphate, phosphate acids
Response to Perturbation in Acid-Base Balance
Overview
- Physicochemical Buffering
- large buffering capacity
- will alter [HCO3-]
- Physiological compensation (open system)
- Resp - alter paCO2
- CO2 crosses cell membranes easily
- rapidly and predictably affects IC pH (mins - hrs)
- Renal - alter HCO3- excretion
- slower (max at 7 days)
Buffers
- A buffer is a solution with the ability to minimise changes in pH when an acid or base is added to it
- A weak acid with its conjugate base
- Most effective at pH of pKa +/- 1 (in a closed system)
| Site | Buffer | Comment |
| ISF | HCO3- PO4- protein | for metab acids too low conc too low conc |
| Blood | HCO3- Hb plasma proteins PO4- | imp for metab acids imp for CO2 minor buffer too low conc |
| ICF | protein PO4- | important (high conc, pKa 6.8) important (high conc, pKa 6.8) |
| Urine | PO4- ammonia | responsible for most of titratable acidity imp - formation of NH4+ |
| Bone | Calcium carbonate | in prolonged metab acidosis |
HCO3- Buffer System
- 80% of ECF buffering
- Henderson-Hasselbach equation
- pH = pKa + log([HCO3-]/(0.03 x pCO2)) where pKa = 6.1 at 37C
- Would not be a good buffer in a closed system at pH 7.4, but in the body the system is open, ie both CO2 and HCO3- concentrations can be manipulated by the lungs and kidneys respectively
Haemoglobin and Protein Buffering
- Buffering by imidazole group of histidine residues, pKa 6.8
- Hb x 6 more important than plasma proteins because:
- x 2 concentration (150g/L vs 70g/L)
- x 3 amount of His per molecule
- deoxyHb more effective buffer than oxyHb → 30% of Haldane Effect
Link Between IC and EC Compartments
- Transfer of CO2
- Ionic Shifts (proton-cation exchange)
| Buffering by | ||||
| ECF | ICF | |||
| metabolic | acidosis | 40% | 60% | Na+ - H+ exchange 36% K+ - H+ exchange 15% other 6% |
| alkalosis | 70% | 30% | ||
| respiratory | acidosis | 1% | 99% | |
| alkalosis | 3% | 97% |
Respiratory Regulation
Important Relationships:
- paCO2 ⍺ VCO2 / VA
- paCO2 - arterial pCO2
- VCO2 - production rate of CO2
- VA - alveolar minute ventilation
- If alveolar minute ventilation halves, then paCO2 will double
- 1mmHg ↑ paCO2 → ↑ VA 2L/min due to increased ventilatory drive
- Eliminates 20,000 mmol /day of volatile acid
Renal Regulation
- Kidneys are the only route of elimination of fixed acids
- Net excretion of 100-150mmol/day of acid
Processes involved
- Reabsorption of filtered bicarbonate (4,000 - 5,000 mmol/day)
- All filtered bicarb is "reabsorbed"
- 90% in PCT
- 10% in asc LoH, DT and CD
- Excretion of titratable acidity. Net excretion of H+ and acid anions (1 - 1.5 mmol/kg/day, ie 70 - 100mmol/day)
- Secretion of H+ ions
- Mainly in distal tubules, once all bicarb has been reabsorbed
- Buffered by HPO42-
- Associated with formation of a new bicarb
- Na+/H+ antiporter can only work down to a certain urinary pH, below which the concentration gradient is too much to pump against. Buffering increases how much H+ can be secreted
- Excretion of ammonia
- NH4+ secreted by proximal and distal tubules
- Associated with formation of new bicarb
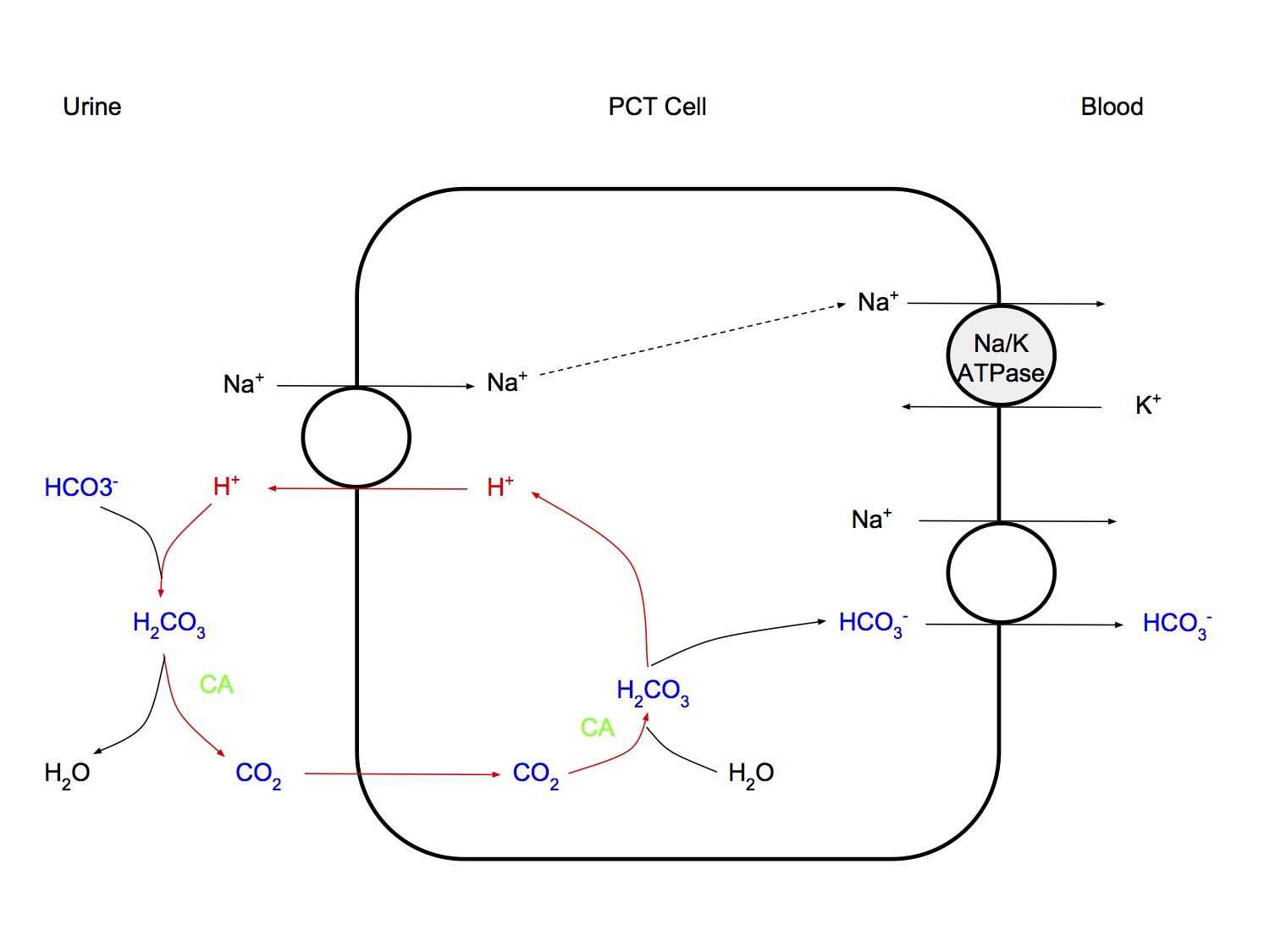
Proximal Tubular Mechanisms
- H+ ions secreted by secondary active Na+/H+ antiporter (Na+ going down conc gradient generated by energy-consuming Na+/K+ ATPase pump)
- Secreted H+ combines with HCO3-, catalysed by carbonic anhydrase → CO2 enters cell, forms new HCO3- → crosses basolateral membrane, going down conc gradient
- This does not result in net excretion of H+ from the body
- "high capacity, low gradient" system
- High capacity - secretes large amt of H+ (4,000 - 5,000mmol/day)
- Low gradient - low pH gradient as tubular pH can be maximally reduced from 7.4 to 7.0 only
- ?due to no net secretion of H+, just loss of bicarb buffering
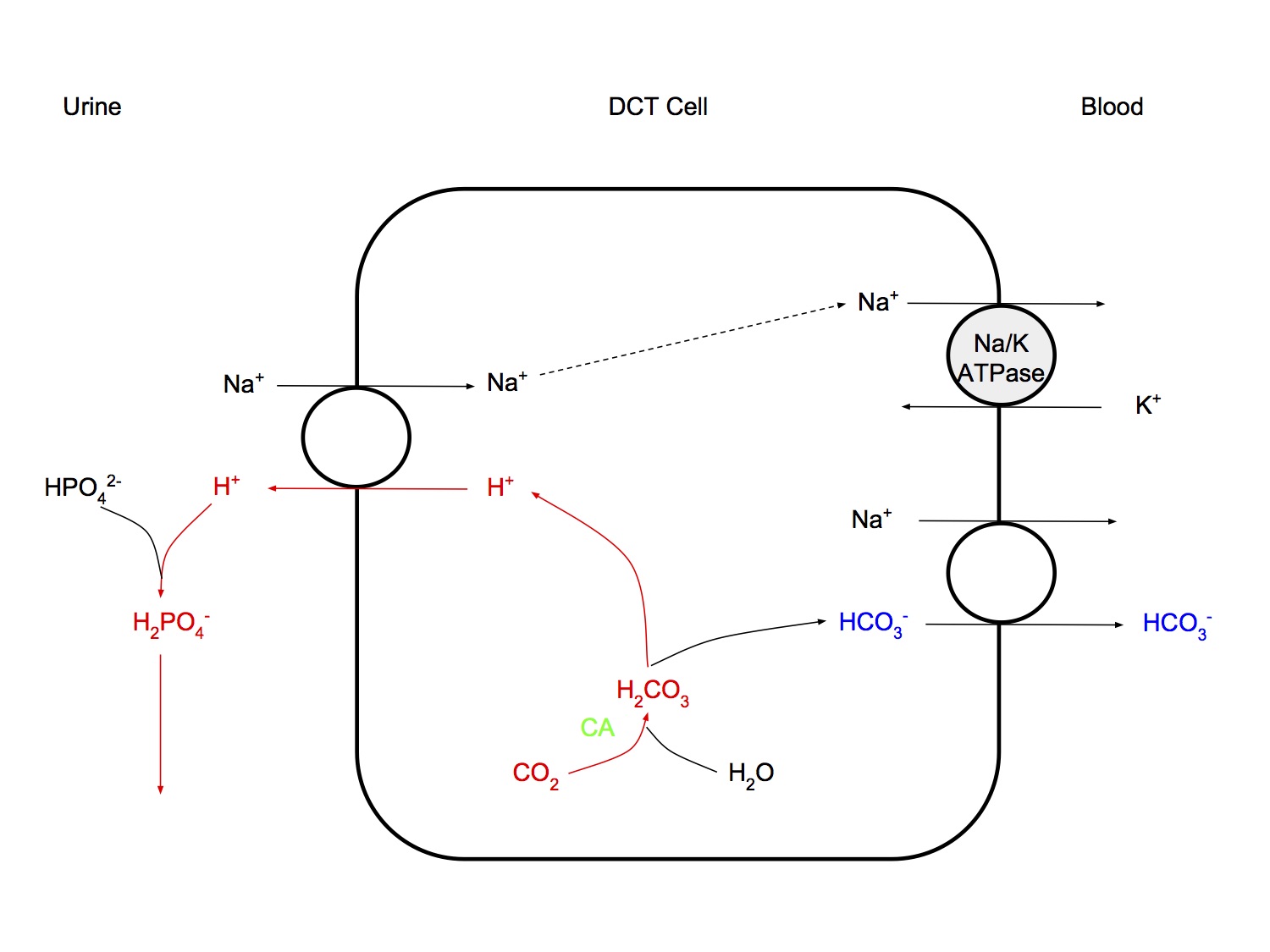
Distal Tubular Mechanisms
- H+ is secreted by intercalated cells, using primary, active transport, a H+-ATPase transporter
- After all bicarb has been reabsorbed, secreted H+ is buffered by filtered buffer HPO4-- ( and other minor role buffers, eg acetoacetate) (titratable acidity)
- Results in net elimination of H+ from the body
- "low capacity, high gradient" system
- low capacity - only 70 - 100 mmol H+ excreted
- high gradient - results in maximally acidic urine pH 4.4
Titratable acidity
- Refers to the H+ ions bound to filtered buffers in the urine (mainly HPO4--)
- The amount of alkali (NaOH) required to titrate the urine to pH 7.4
- Max urine acidity pH 4.4
- NH4+ does not contribute to titratable acidity as pKa is 9.2, hence when urine pH titrated back up to 7.4, no H+ is released from NH4+
Ammonia Secretion
- In PCT, LoH and DT, glutamine is split by glutaminase to form 2 NH4+ and 2 HCO3-
- ie NH4+ formation is associated with new bicarb production
- Traditional teaching is that NH3 is produced and buffers urinary H+, but this is now considered incorrect
- How urinary excretion NH4+ contributes to net loss of acid from the body may be more complex than this and involve the liver and urea formation
Role of the Liver
- CO2 production from complete oxidation of substrates
- Metabolism of organic acid anions (lactate, ketones, aminoacids)
- Metabolism of NH4+
- Production of plasma proteins
Regulation of Intracellular pH
- A stable intracellular pH is important due to
- small ion trapping (Davis Hypothesis)
- effect on macromolecule function
- Stable intracellular pH is maintained by
- Intracellular buffering
- Adjustment of arterial pCO2
- Extrusion of fixed acids from cell
Intracellular Buffering
- Physico-chemical buffering
- Metabolic buffering
- Change in pH → change in enzyme activity → change in metabolism of acids
- eg alkalosis → ↑ production of lactate and other acidic intermediaries
- Organelle buffering
- Sequestration or release of H+ from organelles, that opposes any change in intracellular pH
- eg acidosis → H+ sequestered into mitochondria and used for oxidative phosphorylation
Adjustment of arterial pCO2
- CO2 diffuses through cell membrane easily, thus changes in paCO2 → rapid change in intracellular pH
Extrusion of fixed acids from cell
- Excess acids are produced intracellularly by metabolism
- The H+ ion produced is involved in coupled counter exchange of ions across the cell membrane (H+, Na+, HCO3- and Cl-)
- Process is electroneutral, with no effect on membrane potential
- Results in net loss of fixed acid from intracellular fluid
Acid-Base Disturbances
Respiratory Acidosis
Definition
- An abnormal primary process in which paCO2 rises to a level higher than expected
Causes
- ↓ Alveolar minute ventilation
- Central respiratory depression
- drugs (opioids, sedatives, etc)
- CNS pathology (trauma, tumour, etc)
- Neuromuscular disease
- Myaesthenia gravis
- Guillaine Barre
- Muscle relaxants
- Lung/chest wall pathology
- Airway obstruction
- Aspiration
- CAL
- Inadequate mechanical ventilation
- ↑ CO2 production
- Hypermetabolic states, eg malignant hyperthermia
- ↑ Inspired CO2
- Rebreathing
- Addition of CO2 to inspired gas
Maintenance
- ↑ paCO2 is a potent stimulus for ventilation, hence respiratory acidosis will correct rapidly unless some abnormal factor maintains hypoventilation
Physiological Effects
- Respiratory
- Stimulation of ventilation
- Right shift of ODC
- CVS
- Sympathetic stimulation → periph v/c, tachycardia, sweating
- Periph v/d (direct effect)
- CNS
- Cerebral vasodilatation and increased ICP
- CNS depression at high levels (pCO2 > 100mmHg) - CO2 narcosis
Compensation
- Acute
- Physico-chemical buffering only (99% intracellular)
- Chronic
- Renal compensation - kidneys retain HCO3-
- 3 - 4 days to reach maximal compensation
Assessment (Bedside Rule)
- Acute
- ↑ pCO2 by 10mmHg → ↑ [HCO3-] by 1mmol/L
- Chronic
- ↑ pCO2 by 10mmHg → ↑ [HCO3-] by 1mmol/L
Metabolic Acidosis
Definition
- An abnormal primary process that leads to an increase in fixed acids in the blood
Causes
- Gain of strong acid
- ↑ Anion Gap acidosis
- Loss of base
- HCO3- loss from kidneys or GIT
- Normal AG acidosis
↑ AG Acidosis
- Aspirin and other toxins (ethylene glycol, methanol)
- Blood loss → lactic acidosis (type A), and type B lactic acidosis eg metformin
- Chronic or acute renal failure
- Diabetic ketoacidosis and other causes of ketoacidosis (starvation, alcoholic)
Normal AG Acidosis
- Renal
- Renal tubular acidosis
- Carbonic anhydrase inhibitors (acetazolamide)
- GIT
- Diarrhoea
- Drainage of pancreatic or biliary secretions
Maintenance
- Continues while primary disorder persists
Physiological Effects
- Resp
- ↑ Ventilation
- Right shift ODC
- ↓ 2,3 DPG levels in RBCs → left shift ODC
- CVS
- ↓ Myocardial contractility
- ↑ Sympathetic → ↑ HR, v/c
- Periph v/d (direct effect)
- ↓ Cardiac response to effects of catecholamines
- Venoconstriction
- Pulmonary vasoconstriction
- Associated hyperkalaemia → cardiac effects
- Other
- Hyperkalaemia (shift of K+ out of cells in exchange for H+)
- Bone reabsorption (chronic acidosis)
Compensation
- Hyperventilation
- Onset of respiratory compensation within minutes, well advances at 2 hrs, maximal at 12 - 24hrs
Assessment (Bedside Rule)
- Expected pCO2 = 1.5 x [HCO3-] + 8
Structured Approach to Assessment
Kerry Brandis has an excellent section on his website about assessment of acid-base disorders. His recommended approach is as follows:
Clinical Assessment
- What acid-base disorders are likely given the clinical picture?
Acid-Base Diagnosis
- pH - assess net deviation of pH
- Pattern - check the pattern of pCO2 and bicarb
- Clues - check additional clues from other investigations
- Compensation - assess the degree of compensation
- Formulation - make an acid-base diagnosis
- Confirmation - consider additional tests to confirm the hypothesis
Clinical Diagnosis
- Synthesise all the information together
See here for the full approach
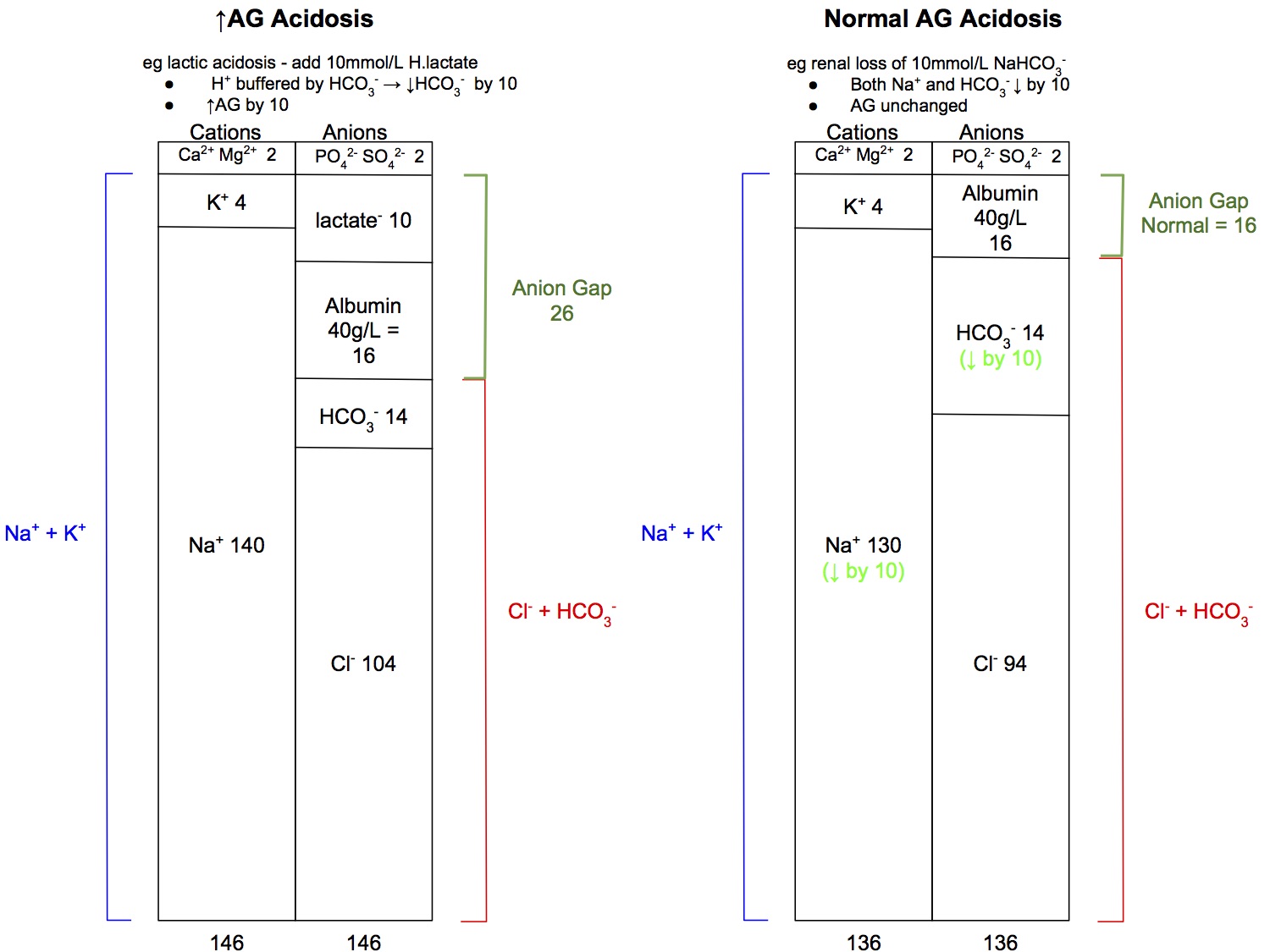
Anion Gap
- AG = ([Na+] + [K+]) - ([Cl-] + [HCO3-])
- Normal AG = 16 (if albumin 40g/L)
- It is useful to calculate the anion gap as
- Helps narrow down the diagnosis of a metabolic acidosis (ie increased vs normal AG acidosis) though in the anaesthetic context, it's usually increased AG
- Helps to exclude a mixed acid-base disorder, ie a second concurrent disorder, if all the numbers "add up"
Anion Gap and Albumin
- The normal AG is 16mmol/L if albumin levels are normal
- Albumin (at 40g/L) provided 16mmol/L of negative charge
- If albumin is low, the body will compensate by retaining more Cl- to maintain electroneutrality, and hence the value of the" normal" AG will reduce
- if albumin 30g/L → normal AG 12mmol/L
- if albumin 20g/L → normal AG 8mmol/L
- Thus, if the patient's albumin is 20g/L, with a calculated AG of 16, this actually suggests an increased AG acidosis is present
↑ AG Acidosis
- Aspirin and other toxins (ethylene glycol, methanol)
- Blood loss → lactic acidosis (type A), and type B lactic acidosis eg metformin
- Chronic or acute renal failure
- Diabetic ketoacidosis and other causes of ketoacidosis (starvation, alcoholic)
Normal AG Acidosis
- Renal
- Renal tubular acidosis
- Carbonic anhydrase inhibitors (acetazolamide)
- GIT
- Diarrhoea
- Drainage of pancreatic or biliary secretions
- Normal AG acidosis is caused by loss of HCO3- from the kidneys or GIT
- However, HCO3- is not lost in isolation, it is always lost with the same amount of Na+ (or K+) to maintain electroneutrality, hence the anion gap is unchanged
Do the numbers add up?
- In a simple acid-base disorder, ie only one primary disorder present, one would expect all the numbers to change by the same amount
- eg if there is ketoacidosis with 10mmol/L of ketoacids added to the blood, would expect
- HCO3- to decrease by 10, from 24 down to 14mmol/L due to buffering
- Anion Gap to increase by 10, from 16 to 26mmol/L
- Base excess to decrease by 10, from 0 to -10mmol/L
- The above analysis is a gross oversimplification, as only 40% of buffering is done by HCO3- (see also section on delta ratio) but in practice, it is reassuring when the numbers "add up". If they don't, consider looking for a second concurrent acid-base disorder
Quantitative Acid-Base Analysis
Stewart Approach
There is one Learning Objective on the Stewart approach to acid-base analysis:
BT_PO 1.79 Describe acid-base chemistry using the Henderson-Hasselbach Equation and strong ion difference
The Stewart approach is a quantitative physicochemical approach to acid-base analysis, which is very complex and requires computers to do the calculations. It is a more accurate and comprehensive system of acid-base analysis compared to the oversimplified, yet very easy to use and clinically useful "6 bedside rules" approach.
I find it highly doubtful that a question on it would ever come up in the exam, except perhaps for an MCQ. I would not waste time reading up on it, the yield is too low.
For completion, I include the definitions below:
Strong Ion
- An ion that is always fully dissociated in solution,
- eg Na+, K+, Cl-, lactate
Weak Ion
- Substances that only partially dissociate in solution
- eg weak acids
Strong Ion Difference (SID)
- The sum of all strong cation concentrations minus the sum of all strong anion concentrations
- SID = net charge that must be balanced by charges on weak acids
- SID = ([Na+] + [K+] + [Ca2+] + [Mg2+]) - ([Cl-] + [other strong anions])
You can read Stewart's textbook here if you are really interested and have spare time and are a masochist