Definitions
Beer's Law
The absorption of radiation as it passes through a substance increases exponentially as the concentration of the substance increases
Lambert's Law
The absorption of radiation as it passes through a substance increases exponentially as the distance it travels through the substance increases
Hagen-Poiseuille Equation
- Describes laminar flow of a Newtonian fluid through a cylindrical pipe of constant cross-section
V=Pπ r4/nl
V = velocity
P = driving pressure
r = radius
n = viscosity
l = length
Reynold's Number
Re = 2rvd/n
r = radius
v = velocity
d = density
n = viscosity
Turbulent flow in a straight smooth tube is probable when Re > 2000
Henry's Law
- The amount of gas dissolved in a liquid is proportional to the partial pressure of the gas that is in equilibrium with the liquid, at a constant temperature
Absolute Humidity
- The mass of water vapour present in a given volume of air
- Units: mg/L or g/m2
Relative Humidity
- The ratio of the mass of water vapour present in a given volume of air to the mass of water vapour required to saturate that volume at that temperature
- Units: %
Saturated Vapour Pressure
- The maximum partial pressure of a vapour at a particular temperature, above which condensation occurs
Dew Point
- The temperature to which a gas must be lowered in order for water to condense from it
Latent Heat of Vapourisation
- The amount of heat energy required to change a liquid to the gaseous phase
Gas Laws
Boyle's Law
- The volume of a gas is inversely proportional to the pressure exerted on it, at a constant temperature
Charles' Law
- The volume of a gas is directly proportional to the temperature, at a constant pressure
Gay-Lussac's Law
- The pressure of a gas, kept at a constant volume, is directly proportional to its temperature
Avogadro's Law
- The volume of a gas is directly proportional to the number of molecules present
Ideal Gas Law
- PV = nRT
- P - pressure
- V - volume
- n - number of moles
- R - universal gas constant
- T - temperature (Kelvin)
Gas Flow
Laminar Flow
- Flow occurs in parallel streams within the tube
- The central stream has velocity double the average velocity
- Described by the Hagen-Poiseuille equation:
V=Pπ r4/nl
V = velocity
P = driving pressure
r = radius
n = viscosity
l = length
Turbulent Flow
- At higher flow rates, disorganised turbulent flow occurs
- P = kV2
- Pressure is proportional to the square of the velocity
- Viscosity is relatively unimportant, but density becomes a factor
Whether flow is laminar or turbulent is predicted by Reynolds No:
Re = 2rvd/n
r = radius
v = velocity
d = density
n = viscosity
Turbulent flow in a straight smooth tube is probable when Re > 2000
Pulse Oximetry
Principles
- Pulse oximeter is a non-invasive monitor that gives a continuous measurement of arterial functional saturation of haemoglobin
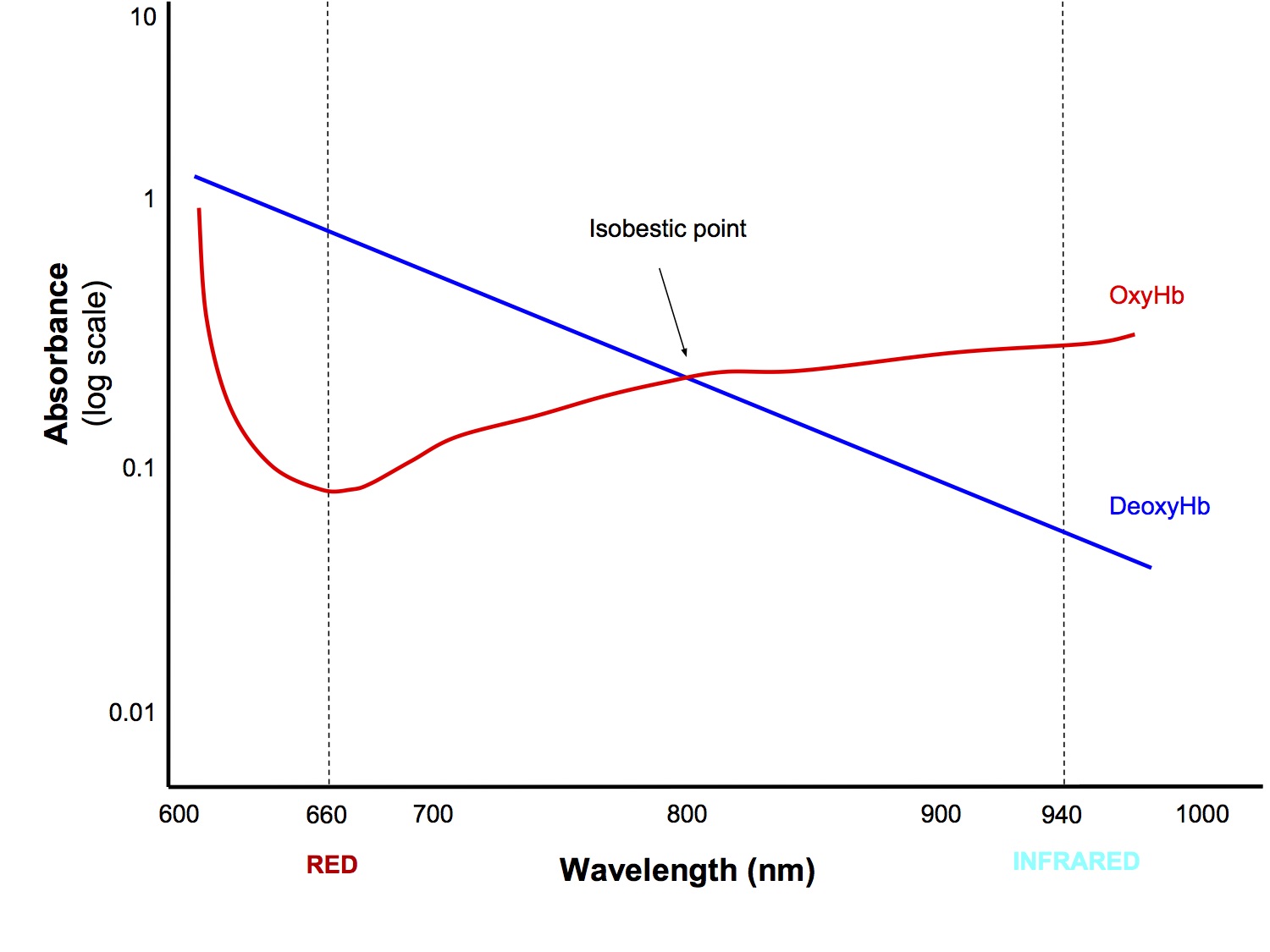
- LEDs emit red (wavelength 660nm) and infrared (940nm) light and the amount that traverses the body part is measured by the detector
- The amount of each wavelength of light absorbed depends on:
- Concentration of the light absorbing substance (Beer’ s Law)
- Length of the light path in the absorbing substance (Lambert’ s Law)
- Ratio of oxyHb to deoxyHb (they have differing absorbance profiles)
- Isobestic Point - the wavelength at which oxy and deoxy Hb absorb the same proportion of the two wavelengths of light (not really clinically relevant)
- The processor calculates the red:infrared absorbance ratio and correlates that against a bank of empirically derived data to provide a SpO2 reading
Beer's Law:
- The absorption of radiation as it passes through a substance increases exponentially as the concentration of the substance increases
Lambert's Law:
- The absorption of radiation as it passes through a substance increases exponentially as the distance it travels through the substance increases
How it works
- One side of clip has high intensity LEDs, emit monochromatic light, wavelength 660nm (red) and 940nm (infrared)
- Other side has a photodetector which detects the light passing through the body part
- 3 phase cycle at > 200Hz:
- Red LED on, infrared LED off
- Red LED off, infrared LED on
- Both LEDs off - so that interference from ambient light can be detected and subtracted from analysis
- Both wavelength are absorbed by HbO and Hb, but at varying proportions
- The amount of each wavelength of light absorbed depends on:
- Concentration of the light absorbing substance
- Length of the light path in the absorbing substance
- Ratio of oxyHb to deoxyHb
- Two wavelengths need to be analysed because if only one wavelength was analysed, could not differentiate whether a change in absorbance was due to change in:
- Overall Hb conc
- oxy to deoxyHb ratio
- Thickness of tissue the light is passing through
- Computer algorithm isolates and analyses only the dynamic component of light absorbance change vs time
- During systole - dynamic ↑ in light absorbance is due to arterial pulse wave
- due to ↑ Hb conc and ↑ artery diameter of arterial vessels
- During diastole - static, baseline absorption by arterial and venous blood, muscle, skin
- By subtracting the static baseline absorbance, only absorbance due to arterial blood is analysed
- The dynamic component is only 2% of the overall signal, ie poor signal strength
- The ratio of absorption of the two wavelengths is calculated and can derive SpO2 using algorithm derived from healthy volunteers
- This corrects for:
- Light scatter - which partially invalidates the Beer-Lambert Law
- The fact that light is passing through tissue other than Hb/HbO
- Empirical data has only been obtained down to 70% saturation in healthy volunteers, so below 70% the data is extrapolated and readings are less accurate
Pros
- Safe, non-invasive
- Accurate (+/- 2%) in range 70-100% in SR
- Fast response time, continuous reading
- Changes in audio tone with change in saturation → allow faster detection of change without needing to look at monitor
- Plethysmography trace gives an index of peripheral perfusion
- Accuracy not affected by presence of HbF or HbS
Cons
- Potential for burns if there is a fault
- Susceptible to errors that lead to inaccurate readings
Sources of Error
- Poor perfusion
- Requires adequate pulsations to distinguish light absorbance by arterial blood from non-arterial tissue
- ↓ perfusion → ↓ signal strength → ↓ accuracy (part of the low signal-to-noise ratio group, but prob deserves its own heading)
- eg
- hypotension
- hypothermia
- proximal BP cuff inflation
- Arrhythmias and aortic balloon pump
- Irregular rhythms and aortic balloon pulsations can confuse the processor and result in inaccurate or no reading
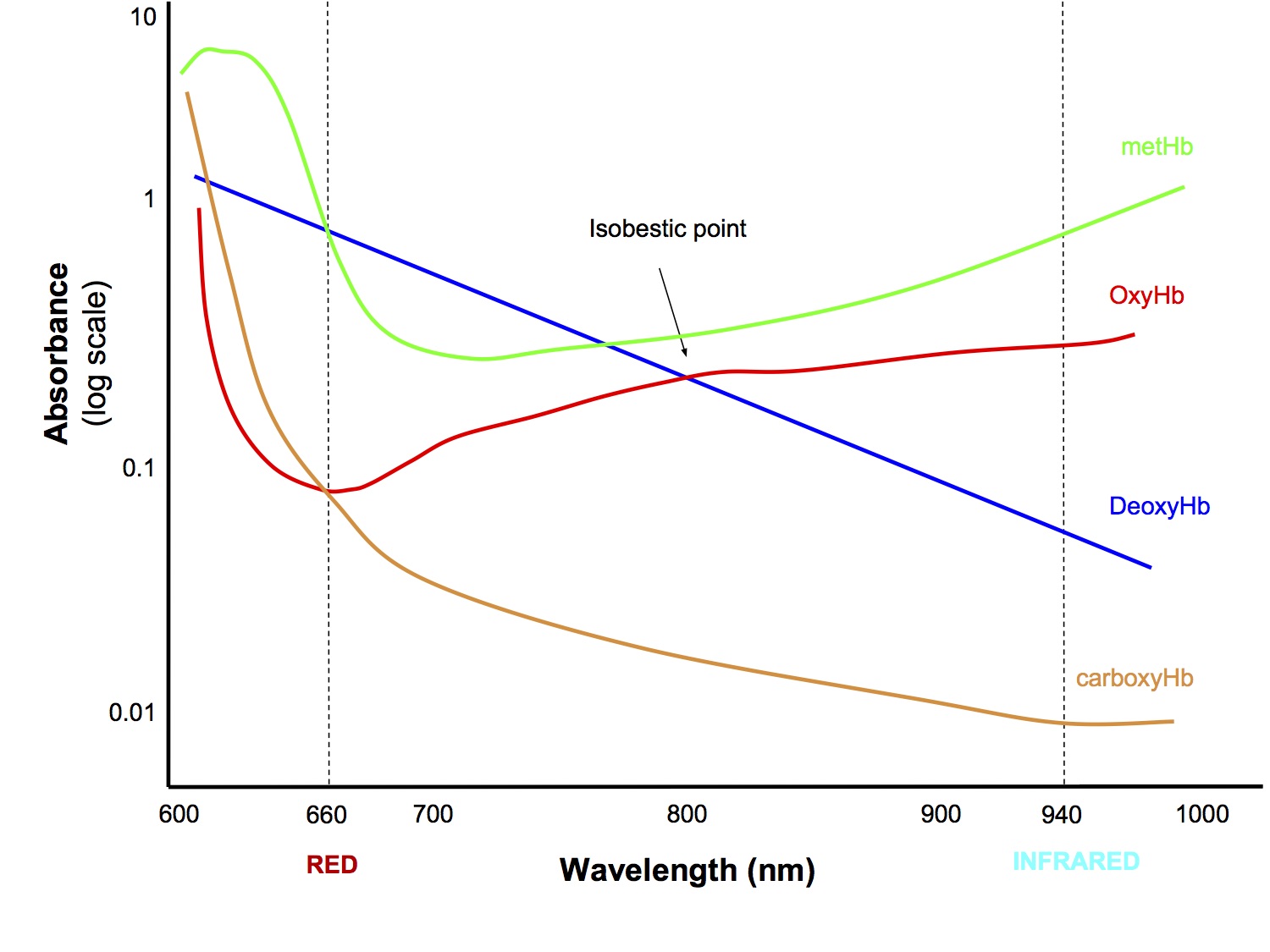
- Different haemoglobins
- Methaemoglobin - absorbs red and infrared light at a ratio of 1:1, which is interpreted by the algorithm as a saturation of 85%, resulting in falsely low readings above 85% and falsely high reading below 85%
- Carboxyhaemoglobin - results in falsely high SpO2 compared to the true fractional SaO2 (see section below)
- Causes of low signal-to-noise ratio
- If there is only small signal from arterial pulse → ↓ accuracy
- eg
- Malpositioned probe → allows optical shunting (light reaches detector without passing through arterial blood)
- Movement artefact → inaccurate analysis of pulsations
- Ambient light
- Electromagnetic interference
- Electrical equipment such as diathermy may induce currents in the wires of the pulse oximeter, which is interpreted as a signal from the light detector
- Coloured dyes and nail polish
- Artefactually ↓ SpO2
- methylene blue
- indocyanine green
- black, blue, green nail polish
- Saturations < 75%
- Due to lack of empirical data for saturations < 75%, estimations are used and the reading is less accurate
- Lag in reading change
- Depending on the settings, the displayed SpO2 reading may lag behind the true SO2 by up to 30 secs
- Venous pulsations
- Prominent venous pulsations may be interpreted as part of arterial pulsation → falsely low SpO2
- May occur with
- low systemic vascular resistance
- high airway pressures with IPPV → phasic venous congestion
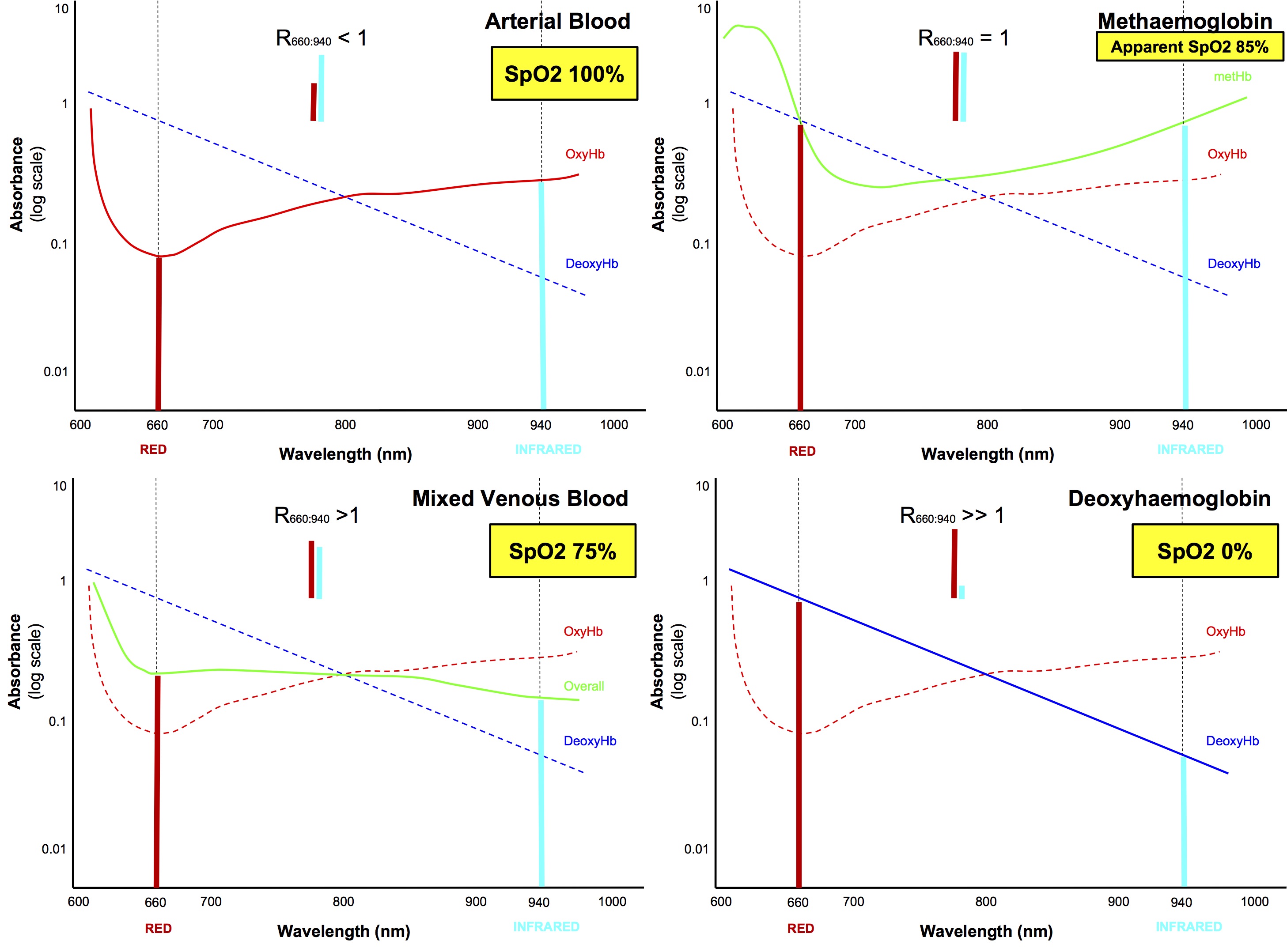
Why does carbon monoxide poisoning falsely elevate SpO2? The absorbance profile looks more like deoxyHb than oxyHb. I'm confused!
- It is confusing because many references state that "the absorbance profile of CO-Hb is similar to that of oxyHb, therefore the pulse oximeter interprets it as oxyHb", which is clearly incorrect if you look at the graphs
- From empirical data, it is known that even in the presence of up to 50% CO-Hb, ie fractional sO2 must be ≤ 50%, pulse oximeters will give spO2 reading > 94%
- I haven't found a good explanation, but it must go beyond the simple shape of the absorbance profiles
- My own rationalisation is as follows:
- Because the y axis is drawn on a logarithmic scale, it is a bit deceptive and distorts our perception
- CO-Hb and deoxyHb have similar absorption ratios, both absorbing roughly 10 x more red light than infrared light, thus if only CO-Hb was present (and no oxyHb) then it would be interpreted as deoxyHb and give an spO2 reading of 0%
- However, at both wavelengths, CO-Hb absorbs roughly 10 times less light than deoxyHb, hence 50% CO-Hb will have the same absorbance effect as 5% deoxyHb
- So in the case of CO poisoning with 50% of haemoglobin as CO-Hb and 50% as oxyHb (assuming 100% functional saturation), the pulse oximeter would sense the presence of what it thinks is 50% oxyHb and 5% deoxyHb, ie interpreted this as saturation of 50/55, ie spO2 91%
- I'm not sure about the validity of this rationalisation, but I'm sticking with it!
- This goes way beyond what you would be expected to know for the exam so don't get stuck on this point, move on!
Capnography
Principles
- Infrared analyser that gives a continuous reading of CO2 concentration
- Gases that have two or more different atoms in the molecule absorb infrared radiation (O2 doesn't absorb IR light)
- Different gases absorb radiation maximally at a characteristic wavelength
- CO2 4.28um
- N2O 4.5um
- By measuring the fraction of radiation absorbed by a gas mixture and applying Beer's Law, the partial pressure of a particular gas can be determined
Device Components
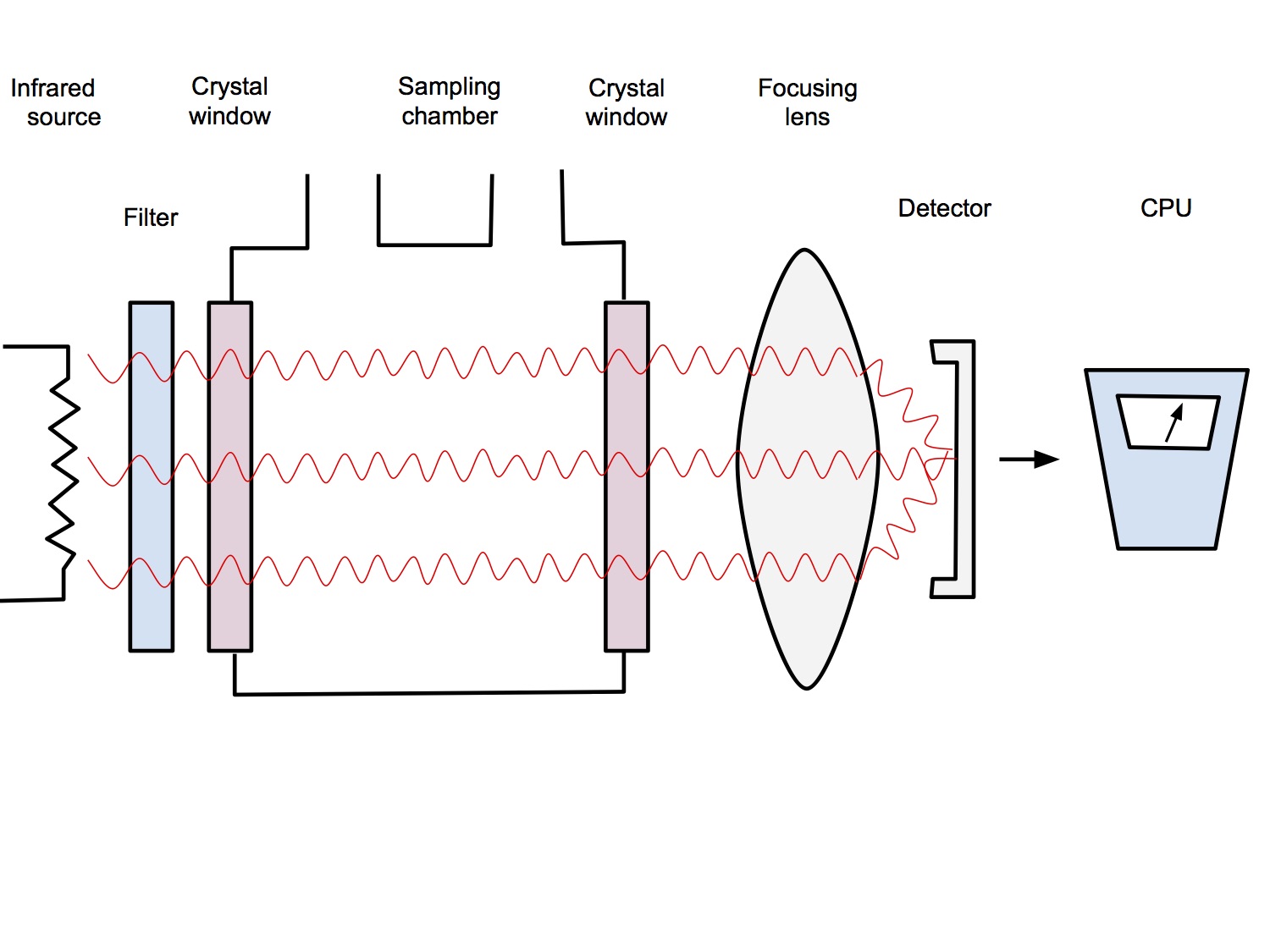
- Infrared source
- IR radiation emitted by a hot wire
- Wavelength filter
- Radiation passes through a filter which allows through only one wavelength of light, or multiple, appropriate wavelengths if it is a multi-gas analyser
- Sample Chamber
- Gas mixture to be analysed passes through the sample chamber
- Sample chamber has windows that are made from sapphire which allows IR light to pass through without being absorbed (glass does absorb IR light)
- Focusing lens
- Focuses light onto the detector
- Detector
- Detects the amount of radiation hitting it and electronically convert that into a gas concentration or partial pressure reading
- Reference chamber
- Variations in the output of the detector could be also caused by
- changes in output of infrared source
- changes in sensitivity of the detector
- variation in transmission of the optics of the system
- To calibrate for this, a second beam of radiation is passed through a reference cell that does not contain CO2
- Any changes not due to changes in CO2 concentration will also be reflected in the output passing through the reference cell and this can be corrected for.
Collision Broadening
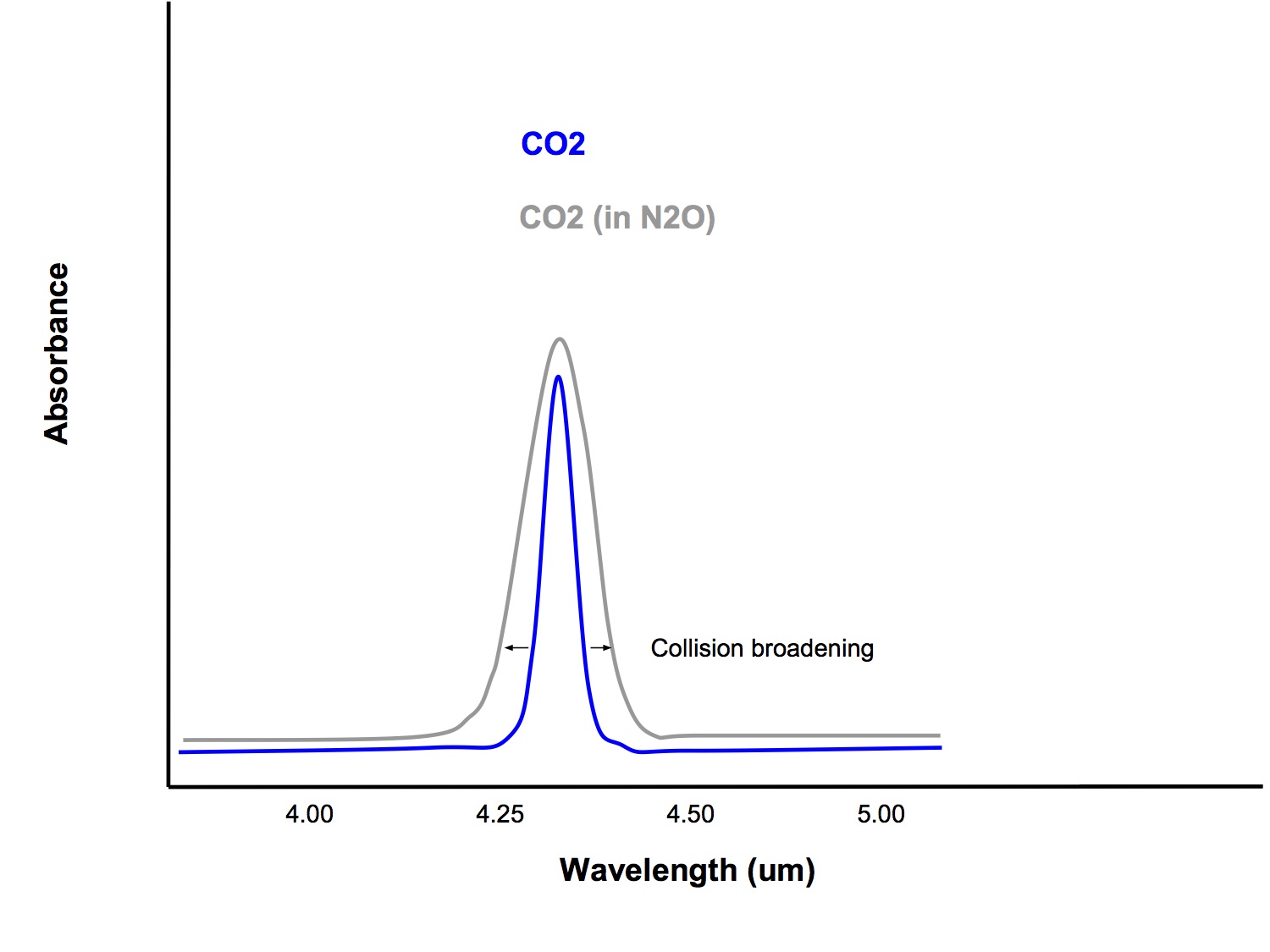
- When gas molecules of different type collide with each other, it slightly alters the way that they absorb infrared light, broadening the band of wavelengths that they absorb.
- Gases such as oxygen and nitrous oxide can affect the amount of infrared light that CO2 absorbs and this is a potential source or error in measurement
- Modern analysers also measure the amount of nitrous oxide and oxygen present and use this information to correct for errors due to collision broadening
Sidestream vs Mainstream
| Sidestream | Mainstream | |
|---|---|---|
| Setup |
|
|
| Response time |
|
|
| Bulkiness |
|
|
| Gas sampling |
|
|
Response Time
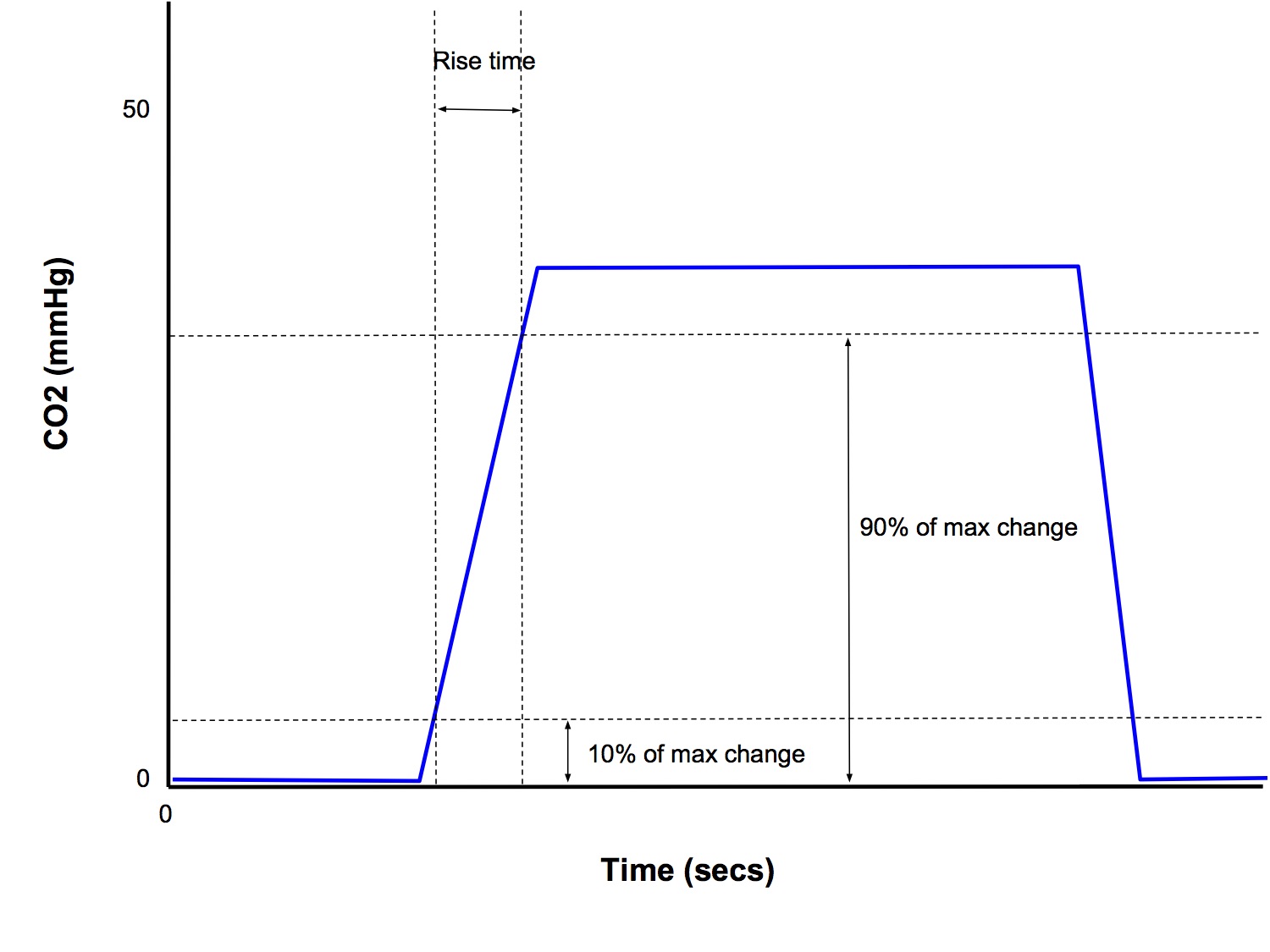
- Response time is the time for an actual change is CO2 concentration to be displayed by the monitor
- Response time = transit time + rise time
Transit Time
- Time taken for the gas sample to move from the sampling site to reach the analyser
- Sidestream - usually < 1sec
- Mainstream - nil (as analyser is at sampling site)
- Transit time can be shortened by
- Using a short, narrow sampling tube
- Using higher suction flow rate in sampling tube
- Using a mainstream analyser
Rise Time
- Time taken for the analyser to respond to a sudden change in CO2 concentration (< 0.4 secs)
- Conventionally reported as the time taken for the measured concentration to rise from 10% to 90% of the final value
- Rise time depends on
- Sample chamber size
- Small chamber size, 1ml → rise time < 0.4sec
- Flow rate
- Too slow → long rise time
- Too fast → significant pressure drop across the sampling line → change the concentration of CO2 → inaccurate reading
Sources of Error
- Errors Related to the Machine
- Nitrous oxide
- The absorption waveband of N2O (peak absorption 4.5um) overlaps with that of CO2 (peak absorption 4.28um), so its presence can cause falsely high CO2 readings
- Contributes to collision broadening
- Collision broadening
- When gas molecules of different type collide with each other, it slightly alters the way that they absorb infrared light, broadening the band of wavelengths that they absorb.
- Gases such as oxygen and nitrous oxide can affect the amount of infrared light that CO2 absorbs and this is a potential source or error in measurement
- Modern infrared analysers provide simultaneous breath by breath analysis of CO2, O2, N2O and the volatile agents. Microprocessor technology can automatically identify the presence of other gases and correct for errors due to collision broadening
- Water condensation
- Water and other liquids have high infrared absorbance and if it enters the measuring chamber can cause erroneous readings
- Mainstream - condensation is minimised by electrically heating the analyser
- Sidestream - a water trap collects condensed water before it reaches the analyser
- Ram-gas effect
- A pressure drop across the sampling line, (eg from inadequate suction along an extended sampling tube) → ↓ ambient pressure → ↓ partial pressure of CO2 → falsely low reading
- Calibration
- It is essential to calibrate the machine with the background gas and intended sampling system in place.
- Modern CO2 analysers should have a three-point calibration with known concentrations of CO2 at regular intervals
- Frequent calibration checks are necessary to minimize errors from changes in atmospheric pressure
- Errors Related to Sampling
- Dilution of sample by fresh gas flow
- Sampling of fresh gas flow during expiration may inadvertently occur if gas sampling rate is too high, particularly with
- smaller tidal volume
- faster respiratory rate
- eg in neonates and infants
- Sampling line disconnection
- Total disconnection of the sampling line will result in analysis of only the ambient air
- Loose connection may allow entrainment of ambient air and dilution of CO2 concentration
- Errors Related to the Patient
- During prolonged expiratory pauses, cardiogenic oscillations can cause oscillations in the recorded CO2 concentration as gas sampling alternates between expiratory gas and fresh gas flow
- In patients with asthma or CAL, end tidal CO2 may under-read, especially if the response time is slow
Humidity
Absolute vs Relative Humidity
Absolute humidity
- The mass of water vapour present in a given volume of air
- Units: mg/L or g/m3
Relative humidity
- The ratio of the mass of water vapour present in a given volume of air to the mass of water vapour required to saturate that volume at that temperature
- Units: %
Humidity in the Airways
- Inspired gas starts to be humidified as it passes through the nose and is fully saturated by the time it reaches the carina
- Absolute humidity 44mg/L (100% relative humidity at 37C)
Humidifiers
Problems with bypassing the humidification function of the nose by intubation
- Dehydration of the mucosa
- Altered ciliary function
- Inspissation of secretions → obstruction, atelectasis, V/Q mismatch
- Loss of body heat, especially neonates
Types of Humidifiers
- Passive - heat and moisture exchangers (HMEs)
- Active - eg THRIVE
How does an HME work?
- The filter comprises of pleats of paper that provide a large surface area on which condensation/vaporization can occur
- The paper is coated with a hygroscopic chemical (calcium or lithium chloride) that adsorbs water and augments the amount of moisture retained
- May also incorporate a hydrophobic layer that further reduces the amount of moisture lost in expiration
- Patient expires warm, fully saturated air
- As the gas passes through the HME it loses heat to the cooler ambient temperature and drops below the dew point, causing condensation - this is the main reason condensation occurs, the hygroscopic and/or hydrophobic properties are secondary!
- As the water condenses, it releases the latent heat of vaporization thus heating the air in the HME
- As the patient inspires dry cool air, it passes through the HME and is heated and as the water vapour pressure is below SVP, evaporation of the condensed water occurs, thus increasing the humidity of the inspired gas
Disadvantages of using an HME
- Increased resistance (require 5-10cmH2O pressure support to compensate for this)
- Increased apparatus dead space (up to 100ml)
- Potential for unrecognized cause of airway obstruction if filter blocks
- Not as effective at heating and humidifying vs active humidifier