Definitions
Tachyphylaxis
- The phenomenon whereby responsiveness to a drug diminishes rapidly after repeat administration, requiring higher doses to achieve the same effect
- Potential mechanisms
- depletion of mediators, eg GTN, ephedrine
- down regulation of receptors eg β -receptors, depolarising neuromuscular junction blockers
Desensitisation
- Synonymous with tachyphylaxis
- The phenomenon whereby responsiveness to a drug diminishes rapidly after repeat administration, requiring higher doses to achieve the same effect
Idiosyncrasy
- An idiosyncratic drug reaction is one that occurs rarely and unpredictably amongst a population
- AKA Type B reaction
- Not related to the drug's primary pharmacological actions
- Not related to drug dose
- May be due to genetic difference in metabolism or immune-mediated
- Includes all hypersensitivity reactions
Isomer
- Isomers are compounds that have the same molecular (empirical) formula, but different structures, and demonstrate physico-chemical and pharmacological differences
Structural/Constitutional Isomer
- Compounds with the same empirical formula, but whose atoms are connected in a different sequence
Tautomer
- Compounds in which, under differing conditions eg pH, the substituent groupings may alter their position
- Structural isomers that readily convert from one isomeric form to another and hence exist in equilibrium
Stereoisomer
- Compounds which have the same empirical formula and whose atoms are attached in the same sequence, but differ in the spatial arrangement of the atoms
Enantiomer
- Stereoisomers that are non-superimposable mirror images (like left and right hands)
- Contain a chiral centre (an asymmetric carbon with four different groups attached to it)
Diastereoisomer
- Stereoisomers with different orientation of substituent groups on either side of a rigid bond (eg double bond or ring structure)
- Alternatively, can be thought of as isomers with two chiral carbons
Bioavailability
- The proportion of drug that passes into the systemic circulation after administration, taking into account both absorption and local metabolic degradation
Isomers
Definition
- Isomers are compounds that have the same molecular (empirical) formula, but different structures, and demonstrate physico-chemical and pharmacological differences
Classification
Isomers can be confusing because there seems to be discrepancies between different texts as to the terminology and classification. The classification system below seems the most logical to me, but be aware that other references may disagree with it.
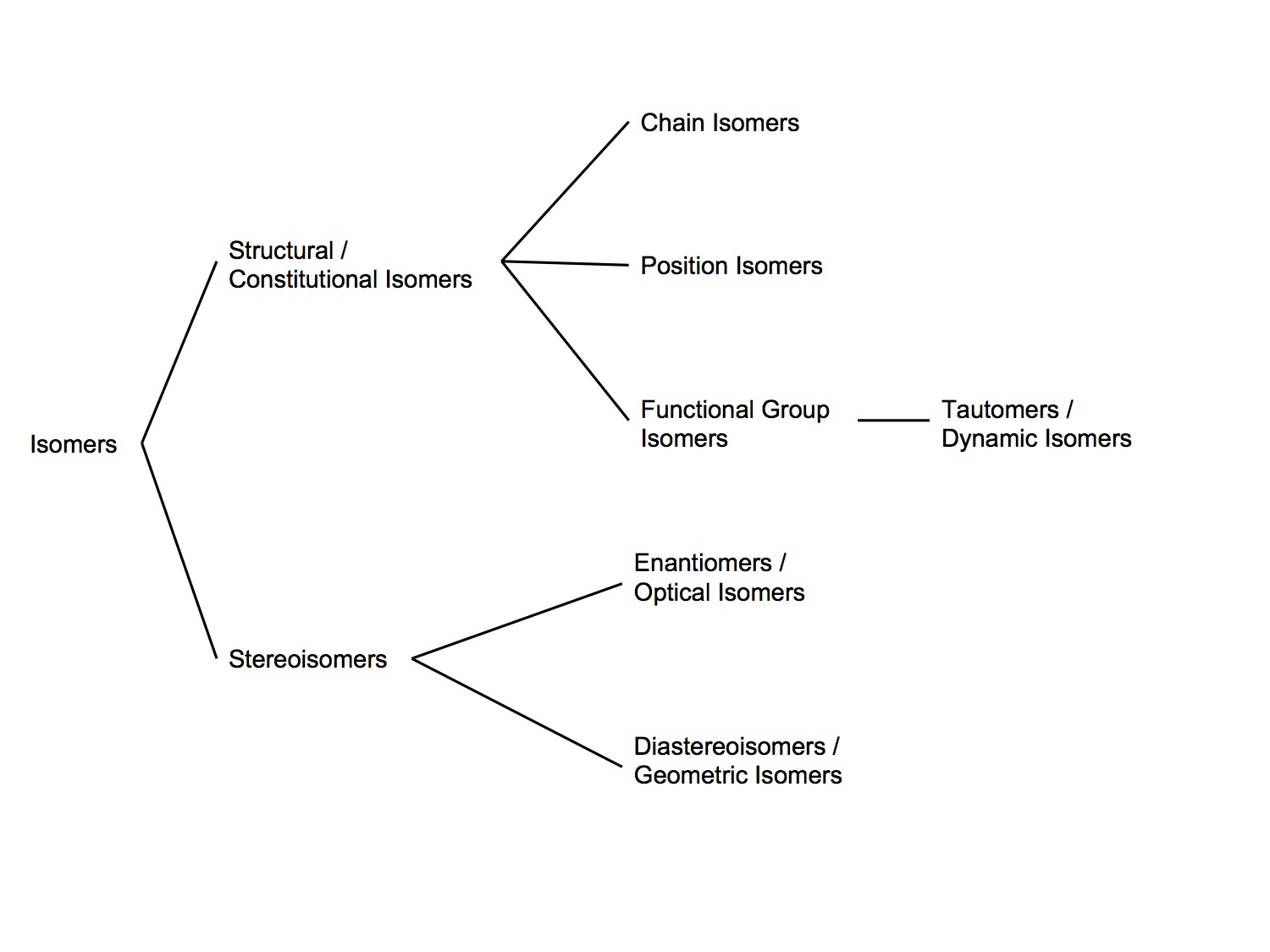
Structural/Constitutional Isomers
- Compounds with the same empirical formula, but whose atoms are connected in a different sequence
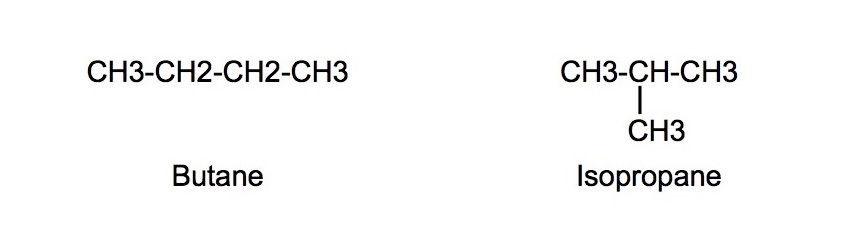
Chain isomers
- Have different branching patterns of carbon chains
Position isomers
- The functional group is located on different carbons in the chain
- Tend to have similar chemical properties
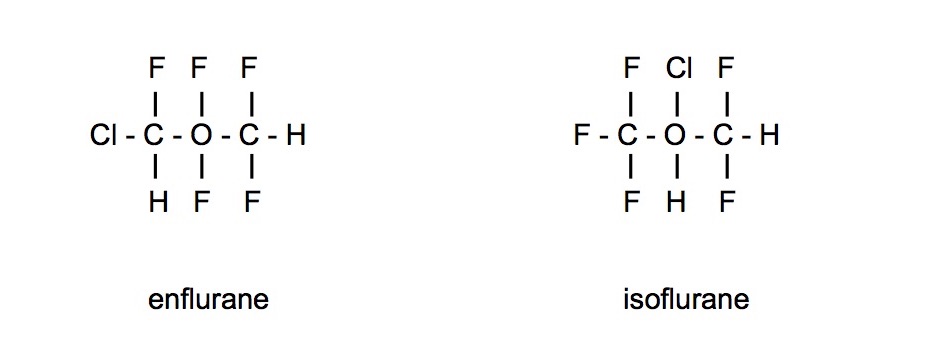
Functional group isomers
- Have different types of bonds and hence different functional groups
- Tend to have very different chemical properties
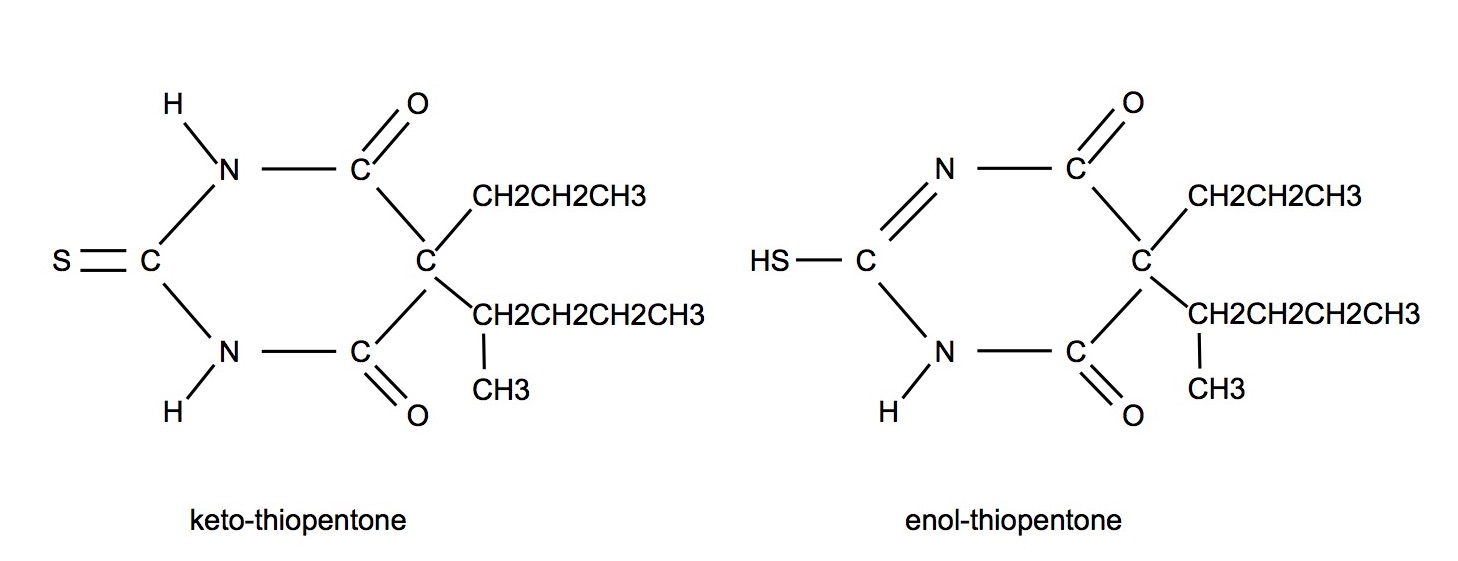
Tautomers/Dynamic Isomers
- Compounds in which, under differing conditions eg pH, the substituent groupings may alter their position
- Structural isomers that readily convert from one isomeric form to another and hence exist in equilibrium
- eg midazolam and thiopentone
Stereoisomers
- Compounds which have the same empirical formula and whose atoms are attached in the same sequence, but differ in the spatial arrangement of the atoms
Significance
- May have marked pharmacokinetic and pharmacodynamic differences eg levo-bupivacaine is less cardiotoxic than dextro-bupivacaine, only levo isomer of morphine has opioid activity
- Different 3 dimensional arrangement → different ability to interact with receptors, enzymes and non-specific binding sites
- Isomer-specific ability of a drug to produce a pharmacological effect is evidence supporting the presence of receptors
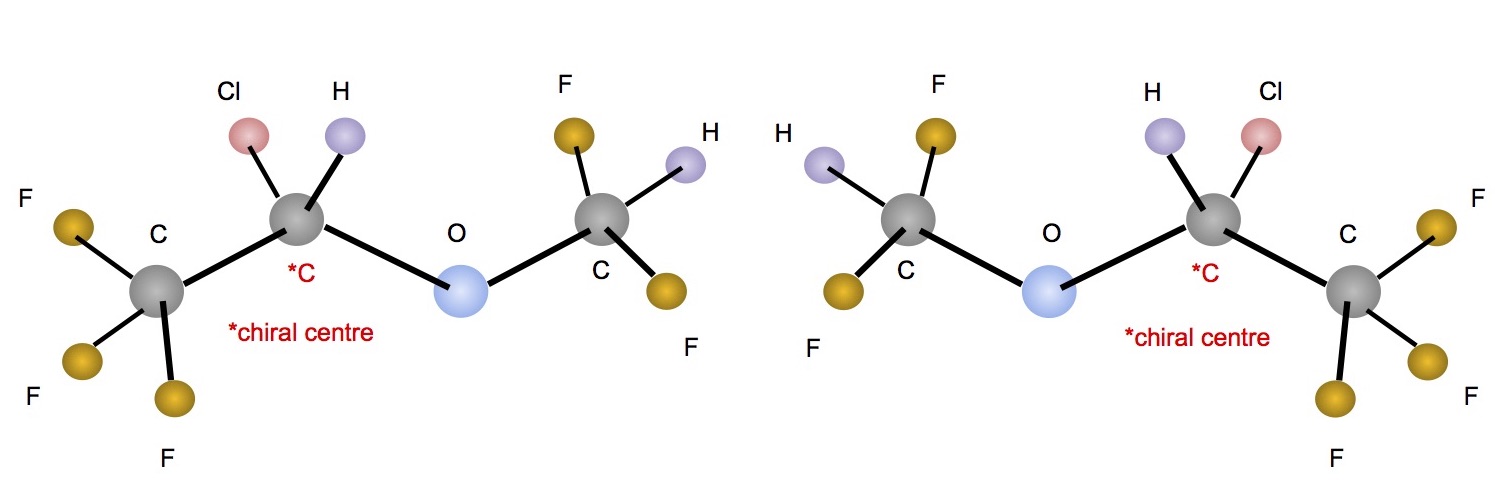
Enantiomers
- Stereoisomers that are non-superimposable mirror images (like left and right hands)
- Contain a chiral centre (an asymmetric carbon with four different groups attached to it)
- Identical physical properties, except the direction in which they rotate polarised light
Classification Systems
Rotation of Polarised Light
- left: levorotatory, l- (-)
- right: dextrototatory, d- (+)
- Racemic mixture contains equal amounts of levo and dextro isomers and therefore has no overall rotating effect on polarised light
Cahn Ingold Prelog Convention
- Ligands around the chiral carbon are assigned a priority based on their atomic number (higher atomic number = higher priority)
- Rectus (R-) priorities increase in clockwise direction
- Sinister (S-) priorities increase in anti-clockwise direction
- Not important to know the exact priority rules. Note that this system has nothing to do with rotation of polarised light and therefore classification in one system does not always correspond to the same classification in the other
- “ Coincidentally” , this seemingly arbitrary nomenclature (R, S) are the initials of Mr R. S. Cahn.
D- and L- Classification
- Simple sugars and amino acids can be classified as D- or L- according to the clockwise or anticlockwise spatial arrangement of COOH, NH3, H and hydrocarbon chain around the chiral carbon
- This is an old classification system, don’ t bother learning it, just be aware of it
- D- and L- are not to be confused with the dextro- and levo- classification system!
Diastereoisomers
- Stereoisomers with different orientation of substituent groups on either side of a rigid bond (eg double bond or ring structure)
- Alternatively, can be thought of as isomers with two chiral carbons
Classification systems
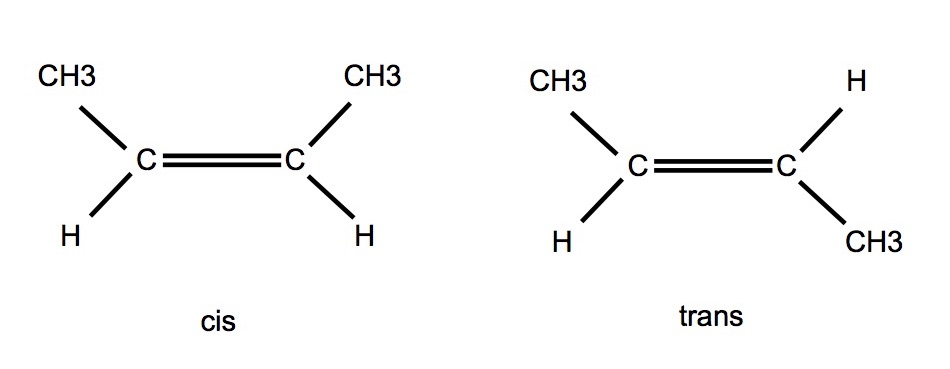
cis- and trans-
- cis- functional groups are on the same side of the double bond
- trans- functional groups are on opposite sides of the double bond
- eg mivacurium is presented as a mixture of isomers:
- trans-trans 60%
- cis-trans 30%
- cis-cis 10%
Other Systems
- Include syn-/anti- and Z-/E- (don't bother about this crap)
Pharmaceutics
Pharmaceutics for the Anaesthetist
- Everything you need to know about pharmaceutics for the exam is contained in this excellent article Pharmaceutics for the anaesthetist by Dr R MacPherson, Anaesthesia 56, 10, pp 965-970 Oct 2001
- It is available through the ANZCA Library