Definitions
CC:CNS Ratio
Ratio of the dose that causes Cardiovascular Collapse to the dose that causes CNS toxicity
Eutectic Mixture
A mixture of substances (in fixed proportions) that has a single melting point that is lower than the melting point of either constituent alone
Minimum Effective Concentration (Cm)
The minimum concentration of a local anaesthetic at the nerve fibre that will block conduction of the action potential
Structure-Activity
Pharmaceutics
- LAs are poorly water soluble
- Formulated as water-soluble hydrochloride salts (acidic)
- Preservative metabisulphite can cause arachnoiditis
- Use only preservative-free LAs for neuraxial blocks
Structure-Activity Relationships
Classification
- Local anaesthetics are weak bases (pKa > 7.4) that are mostly ionized at physiological pH
- They can be classified by
- the type of link joining the lipophilic and hydrophilic ends
- amide
- ester
- the duration of action
- short - lignocaine
- long - bupivacaine, ropivacaine
Ester LAs
- Higher incidence hypersensitivity
- Less stable → shorter shelf life
Amide LAs
- All amide LAs have two 'i's in name
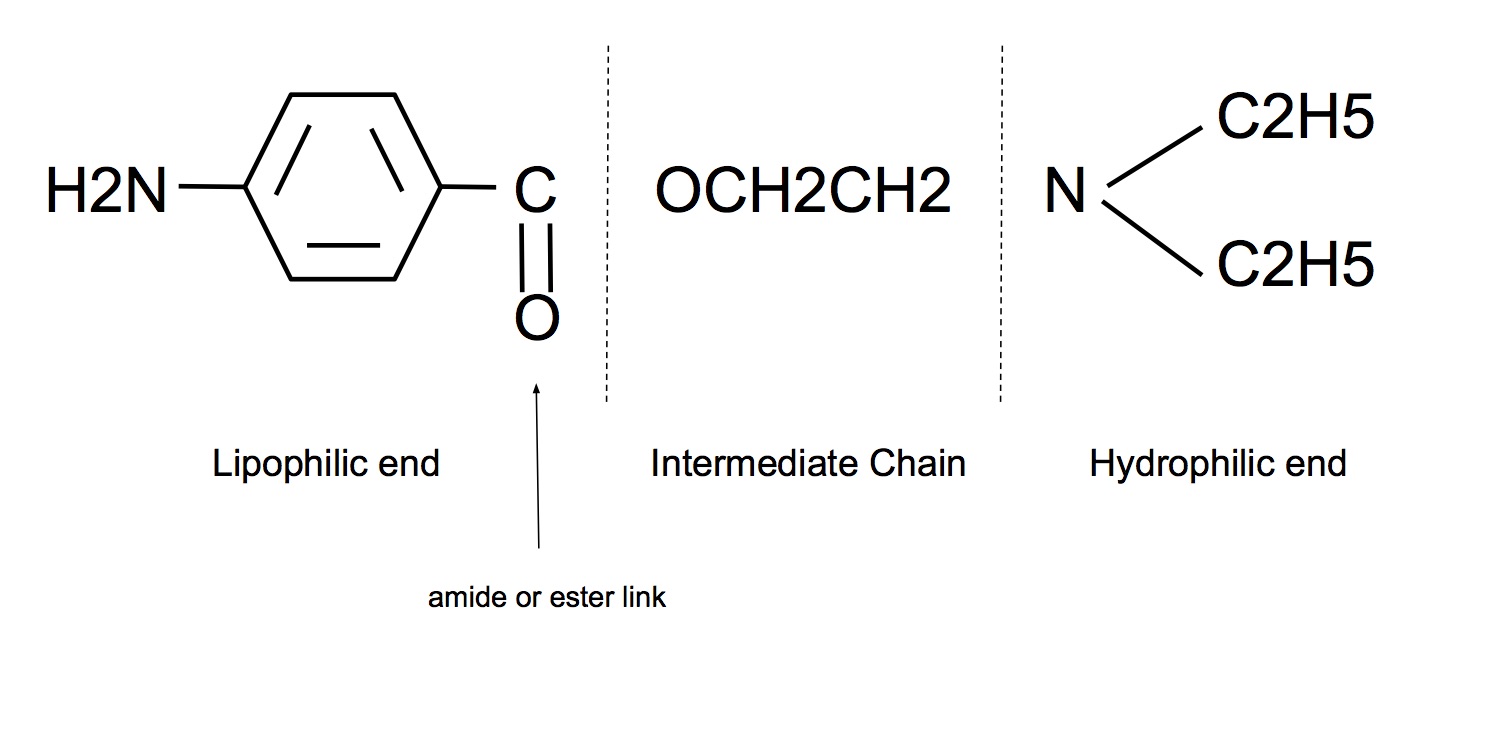
Lipophilic end:
- Unsaturated aromatic ring
- Essential for anaesthetic activity
Hydrophilic end:
- Contains a tertiary amine group that acts as a proton acceptor, ie weak base
- Ionised form is the active for of the drug
Intermediate chain:
- Hydrocarbon chain linked to aromatic ring by either ester (-CO.O-) or amide (-NH.CO-) bond
- Type of link determines
- Stability of drug (esters metabolised more rapidly than amides)
- amides have longer duration of action (hepatic metabolism by amidases)
- amides have longer shelf life
- Propensity for allergy
- esters are hydrolysed to para-aminobenzoate (main metabolite that is assoc with hypersensitivity reactions)
- ↑ Molecular weight
- ↑ potency
- ↑ lipid solubility (main determinant of potency and duration of action)
- ↑ protein binding (but slows onset of action)
- ↑ Degree of alkyl substitution on aromatic ring or rest of structure
- ↑ lipid solubility
- eg Change methyl to butyl group on amino group converts mepivacaine to bupivacaine → ↑ lipid solubility x 26
- eg shortening butyl group to propyl group converts bupivacaine to ropivacaine → ↓ lipid solubility x 4.5
- Isomerism
- LAs are enantiomers, except lignocaine which is achiral
- Have different pharmacodynamic and pharmacokinetic profiles because of differences in interaction with voltage-gated sodium channels
- Bupivacaine is presented as a racemic mixture. Ropivacaine and levobupicaine are pure S-enantiomers
- R-enantiomers have greater potency for blocking both neuronal and cardiac Vg Na+ channels, hence higher potential for therapeutic efficacy as well as systemic toxicity
- S-enantiomers have tendency to block neuronal > cardiac Vg Na+ channels, hence lower potential for systemic toxicity
Potency
- Closely related to lipid solubility
- Lipid solubility correlates to affinity for Vg Na+ channels
- Also affected by intrinsic vasodilator properties and distribution in tissues
Lipid Solubility
- Primary determinant or potency and duration of action
- Lipid solubility correlates to affinity for Vg Na+ channels
- Duration is prolonged as sequestration of more lipid soluble LA within myelin and surrounding perineural compartments → ↓ vascular absorption and uptake, providing a reservoir for slow release of LA
Protein Binding
- LA binds to both tissue and plasma proteins (albumin and α1 acid glycoprotein)
- Degree of plasma protein binding correlates to protein binding onto extracellular axonal membrane
- Highly protein bound LAs are removed from the nerve at a slower rate by vascular uptake → ↑ duration of action
Ionisation
- The primary site of action for LAs is on intracellular side of Vg Na+ channels and charged form is the predominant active form
- pKa correlates to speed of onset because diffusion across the lipid bilayer of axonal membrane by the neutral lipid-soluble form is the primary mechanism of reaching the binding site
Intrinsic vasodilator activity
- Influences potency and duration of action
- According to Hemmings. Pharmacology and Physiology for Anesthesia, 1st Ed 2013:
- Local anesthetics themselves tend to have bimodal effects on vascular smooth muscle such that vasoconstriction results at lower doses while vasodilation predominates at higher concentrations with some minor differences between individual agents
- According to Peck. Pharmacology for Anaesthesia and Intensive Care:
- Local anaesthetics cause vasodilatation in low concentrations (prilocaine > lignocaine > bupivacaine > ropivacaine) and vasoconstriction at higher concentrations
- I would say in the exam that there is disagreement amongst reference texts
- Cocaine solely v/c due to inhibiting uptake 1 and inhibiting MAO
Comparative Pharmacology
| Classification | Potency | Onset | Duration after Infiltration (min) | Maximum Single Dose for Infiltration (mg) | Toxic Plasma Concentration (μg/mL) | pK | Protein Binding (%) |
| Esters | |||||||
| Procaine | 1 | Slow | 45–60 | 500 | 8.9 | 6 | |
| Chloroprocaine | 4 | Rapid | 30–45 | 600 | 8.7 | ||
| Tetracaine | 16 | Slow | 60–180 | 100 (topical) | 8.5 | 76 | |
| Amides | |||||||
| Lidocaine | 1 | Rapid | 60–120 | 300 | > 5 | 7.9 | 70 |
| Prilocaine | 1 | Slow | 60–120 | 400 | > 5 | 7.9 | 55 |
| Mepivacaine | 1 | Slow | 90–180 | 300 | > 5 | 7.6 | 77 |
| Bupivacaine | 4 | Slow | 240–480 | 175 | > 3 | 8.1 | 95 |
| Levobupivacaine | 4 | Slow | 240–480 | 175 | 8.1 | > 97 | |
| Ropivacaine | 4 | Slow | 240–480 | 200 | > 4 | 8.1 | 94 |
| Classification | Fraction Nonionized (%) at pH 7.4 | Fraction Nonionized (%) at pH 7.6 | Lipid Solubility | Volume of Distribution (L) | Clearance (L/min) | Elimination Half-Time (min) |
| Esters | ||||||
| Procaine | 3 | 5 | 0.6 | 65 | 9 | |
| Chloroprocaine | 5 | 7 | 35 | 7 | ||
| Tetracaine | 7 | 11 | 80 | |||
| Amides | ||||||
| Lidocaine | 25 | 33 | 2.9 | 91 | 0.95 | 96 |
| Prilocaine | 24 | 33 | 0.9 | 191 | 96 | |
| Mepivacaine | 39 | 50 | 1 | 84 | 9.78 | 114 |
| Bupivacaine | 17 | 24 | 28 | 73 | 0.47 | 210 |
| Levobupivacaine | 17 | 24 | 55 | 156 | ||
| Ropivacaine | 17 | 59 | 0.44 | 108 | ||
- Click here to download a Word doc with a table comparing local anaesthetics
- It includes a blank version
- You should memorise the key points, then fill the details into the blank version once per day until you have it memorised
- It's a grind, but it doesn't take that long to do
Pharmacokinetics
ADME
- LAs are weak bases with pKa 8-9, hence < 50% unionised, lipid soluble form at physiological pH
- LAs with pKa closer to physiological pH have more rapid onset of action because optimal ratio ionised:unionised
- Intrinsic v/d activity influences apparent potency and duration
Absorption and Distribution
Absorption into systemic circulation depends on:
- Site of injection
- Dose
- Use of adrenaline
- the LA
Plasma conc determined by:
- Rate of absorption
- Rate of tissue distribution (dependent on lipid solubility)
- Rate of clearance
- Other factors
- age
- cardiovascular status
- hepatic function
- protein binding - inversely related to protein binding
Overall amide LAs are more widely distributed in tissues than ester LAs
Lung Extraction
- Lungs extract lignocaine, bupivacaine and prilocaine
Placental Transfer
- Rate and degree of transfer influenced by plasma protein binding
- Ester LAs don't transfer because rapidly hydrolysed
- Acidosis in foetus (eg prolonged labour) → accumulation of LA by ion trapping
Clearance
Esters
- rapid hydrolysis by non-specific esterases in plasma and liver
Amides
- hepatic metabolism (N-dealkylation) → metabolites often active
- minimal renal elimination
Metabolism
Amides
- Prilocaine > lignocaine > bupivacaine, ropivacaine
- Lignocaine - metabolite also active as anti-arrhythmic
- Prilocaine - metabolite may oxidise Hb → metHb
- Bupivacaine - dealkylation. Bound to a1 acid glycoprotein
- Ropivacaine - higher clearance, hence less risk of toxicity
Esters
- Rapid hydrolysis plasma > liver
- metabolite para-aminobenzoic acid is an antigen that may trigger hypersensitivity reaction
Speed of Onset of Action
The onset of action will occur when concentration of LA at target nerve ≥ minimum effective concentration (Cm) (see section in Pharmacodynamics). The speed will be affected by
Drug Factors
- Fraction unionised
- Only the neutral, unionised form is lipid soluble and can pass through the axon lipid membrane to reach the binding site on the intracellular side of the Vg Na+ channel (m gate)
- LAs with a pKa closer to physiological pH will have a higher unionised fraction, and a faster speed of onset
- Actually, you need an "optimal" unionised to ionised ratio, because, while only the unionised form can reach the binding site, it is the ionised form that is active and binds to the Vg Na+ channel
- Illustrate with example lignocaine pKa 7.9 unionised 25% vs rop/bup pKa 8.1, 15% (at pH 7.4)
- LAs don't work well in infected/inflammed areas because environment is acidic → ↓unionised fraction
- Potency
- Less potent LA has faster onset due to
- Higher dose administered → higher concentration to drive diffusion to site of action (similar to why rocuronium works faster)
- For LAs, potency correlates to lipid solubility and protein binding. Greater binding to extra-axonal proteins slows down entry into axons and retards onset
- llustrate with example, 1ml lig 2% vs equipotent dose of 1ml bup 0.5%. Lig contains x 7 more molecules (4 x concentration, plus lignocaine is a smaller molecule).
- Protein binding
- Greater protein binding slows diffusion of LA to target nerve → slower onset
- Bupivacaine and ropivacaine 95% protein bound vs lignocaine 70% → slower onset
- Lipid solubility
- Sequestration of more lipid soluble LA within myelin and surrounding perineural compartments → slows diffusion and onset
- Bupivacaine and ropivacaine lipid solubility > > lignocaine → slower onset
- Molecular weight
- ↑MW of LA correlates with ↑protein binding, ↑lipid solubility and ↑potency
- Dose/concentration administered
- Faster diffusion to target and faster to exceed Cm
- Pregnancy
- Faster onset due to ↓protein binding by α1 acid glycoprotein and progesterone → ↑sensitivity to LAs
- Neonates
- Faster onset due to ↓protein binding by α1 acid glycoprotein
- Use of adjuvants
- NaHCO3- to ↑pH → ↑unionised fraction → faster onset
Patient Factors
- Frequency dependant blockade
- LA gains access to binding site on Vg Na+ channel only when it is in open state
- More frequent stimulation the nerve → faster onset
- Type of nerve
- Small, unmyelinated fibres blocked faster than large, myelinated nerves
- Sympathetic and sensory block before motor
- Distance of injected drug from target nerve
- LA needs to diffuse to target according to Fick's Law
What's the deal with unionised fraction and lipid solubility?
- It can be a bit confusing that we say that having a higher unionised fraction causes a faster onset because it is lipid soluble - as opposed to the ionised, charged, lipid-insoluble form - and can pass through the cell membrane. But we also say that LAs with higher lipid solubility have a slower onset of action because they get sequestered into the myelin
- When we compare lipid solubility of LAs, we are talking about the unionised forms
- Unionised, uncharged molecules are much more lipid soluble than ionised, charged molecules
- But also, comparing the unionised forms, there are differences in lipid solubility between the different LAs
- So the unionised - and hence lipid soluble - form of a less lipid soluble LA, will have the faster onset!
Duration of Action
A local anaesthetic will be effective at blocking nerve transmission while its concentration at the target nerve is ≥ minimum effective concentration (Cm) (see section in Pharmacodynamics). The offset of action is due to removal of LA from the site of action by vascular uptake, when LA conc falls below Cm. Duration of action is affected by:
Drug Factors
- Protein binding
- Greater binding to extra-axonal proteins slows down rate of removal by vascular uptake and provides a reservoir of slow release of LA
- The degree of plasma protein and tissue protein binding correlate
- Bupivacaine and ropivacaine 95% protein bound vs lignocaine 70% bound, therefore longer duration
- Lipid solubility
- Sequestration of more lipid soluble LA within myelin and surrounding perineural compartments → ↓ vascular absorption and uptake, providing a reservoir for slow release of LA
- Bupivacaine and ropivacaine lipid solubility > > lignocaine
- Molecular weight
- ↑MW of LA correlates with ↑protein binding and ↑lipid solubility
- pKa
- LAs with higher pKa will be "ion trapped" intracellularly. IC pH 6.9 is lower than EC 7.4, hence more of LA will be in ionised, lipid insoluble form and unable to exit through the lipid membrane, thus prolonging duration (while also accounting for slower onset)
- Bupivacaine pKa 8.1 vs lignocaine pKa 7.9
- Dose/concentration administered
- Higher dose/concentration of LA → higher initial conc at target nerve
- Intrinsic vasoactive properties
- LAs tend to cause vasodilatation at lower concentrations and vasoconstriction at higher concentrations, with minor differences between individual agents (NB some textbooks say v/c at low concs and v/d at higher concs!)
- Vasoconstriction will retard removal by vascular uptake
- Use of adjuvants
- Vasoconstrictors
- addition of adrenaline will slow removal
- Synergistic agents
- additive that have been shown to prolong duration of neuraxial block include:
- opioids
- dexmedetomidine
- ketamine
- magnesium
- additive that have been shown to prolong duration of neuraxial block include:
- Vasoconstrictors
- Speed of metabolism
- Faster metabolism of LA → maintain greater concentration gradient between nerve and plasma → faster vascular uptake
- Ester LAs metabolised faster than amide LAs (by plasma esterases) → shorter duration of action
Patient Factors
- Site of administration
- LAs will be removed faster from highly vascular sites
- Speed of removal is faster from:
- intercostal > epidural > brachial plexus > periph nerve > skin infiltration
- Type of nerve being blocked
- Larger diameter nerve fibres have higher Cm
- Cm motor fibre ~2 x Cm sensory fibre
- Hence duration of motor block shorter than sensory block
- Neonates
- Immature hepatic enzymes → slower metabolism and longer duration
Pharmacodynamics
Voltage-Gated Sodium Channels
Clinical Relevance
- Vg Na+ channels are responsible for propagating the action potential - a wave of depolarization - along the cell membrane of nerve and muscle fibres
- Local anaesthetics block Vg Na+ channels in nerve fibres, preventing nerve electrical transmission
- Suxamethonium causes motor end-plate Vg Na+ channels to remain in the inactivated state, leading to muscle paralysis
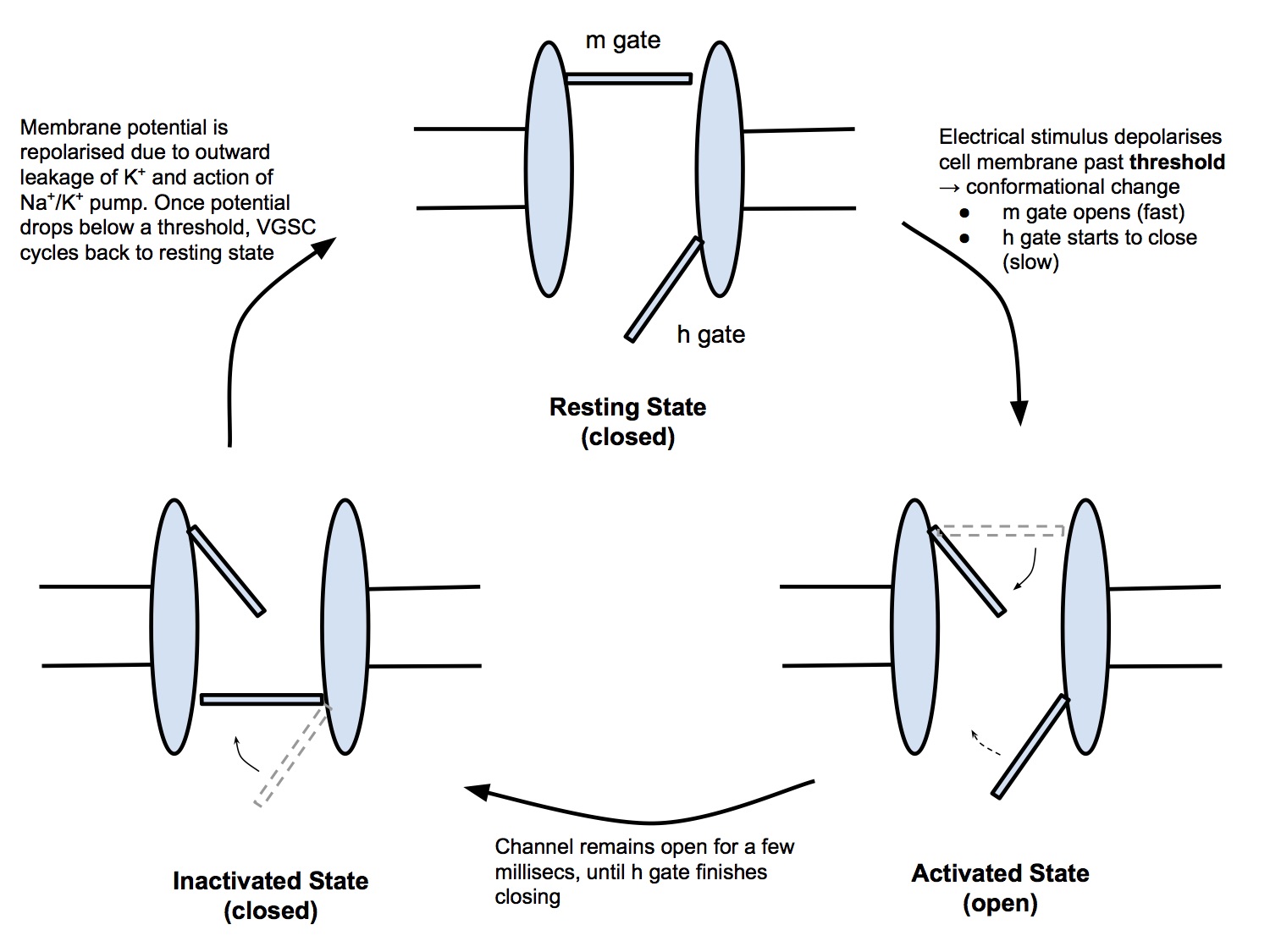
Structure and Function
- Voltage-gated sodium channels - aka fast sodium channels because they open rapidly - are transmembrane channels, that allow Na+ ions to pass intracellularly down its concentration gradient
- Vg Na+ channels open in response to depolarisation of the cell membrane past a triggering threshold and close in response to repolarisation past a triggering threshold
- Conceptually, the Vg Na+ channel is a channel that contains two gates
- m gate (or activation gate)
- h gate (or inactivation gate)
- The Vg Na+ channel exists in 3 states
- Resting state (closed)
- Activated state (open)
- Inactivated stated (closed, refractory)
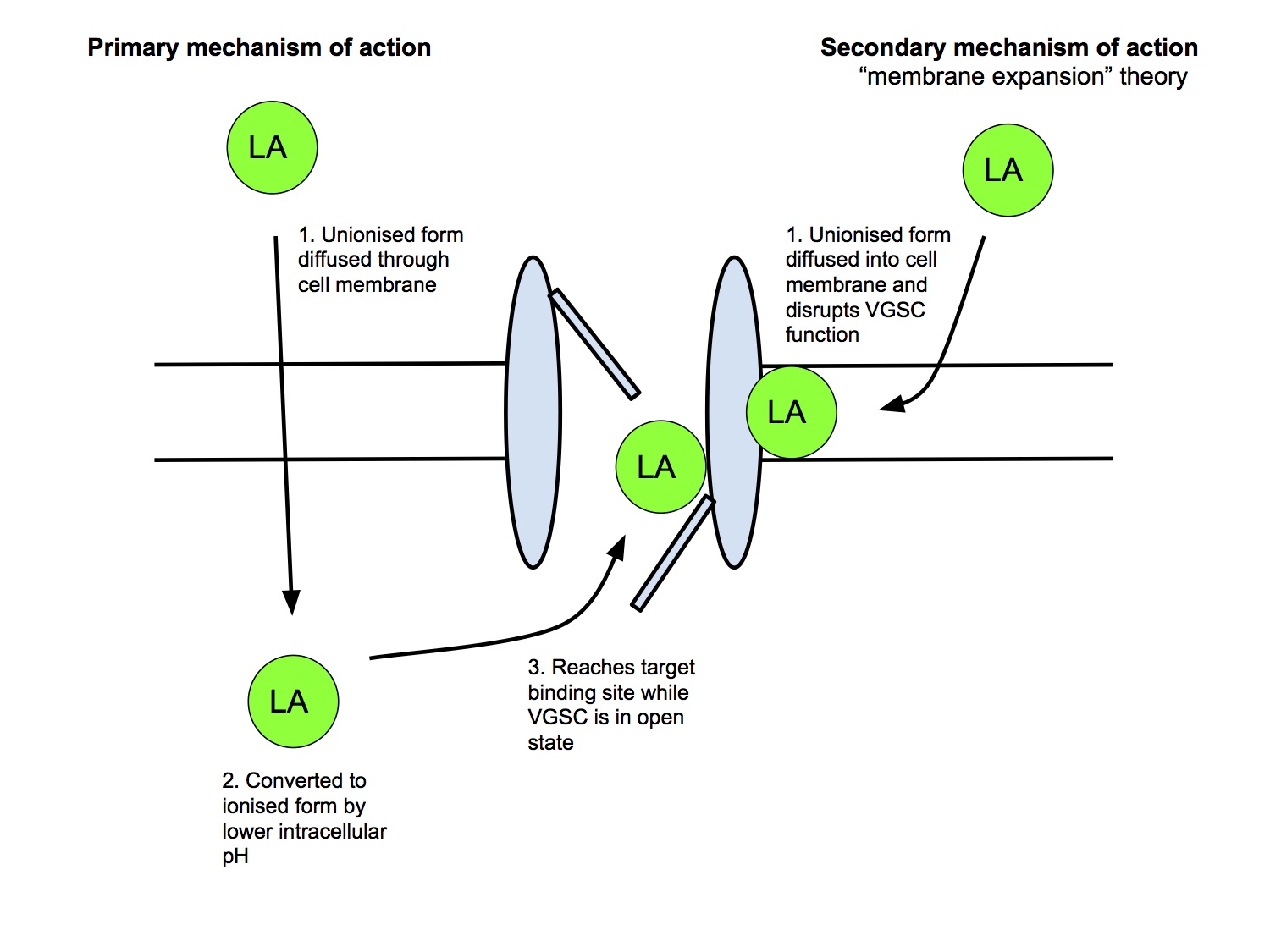
Mechanism of Action
Primary Mechanism
- Unionised, lipid soluble form of LA crosses the cell membrane
- LA converted to the active, ionised form by the lower intracellular pH
- Ionised form binds to binding site (h gate) on intracellular side of VGSC
- LA can gain access to the channel only in open state, hence greater frequency of activity → greater block (frequency dependent block)
- LAs preferentially bind VGSC in the inactivated state, preventing it from cycling back into the resting state, thus preventing further activation
Secondary Mechanism
- Neutral, unionised form can cause membrane expansion and block the Na+ channel (minor effect)
- eg benzocaine, pKa 3.5, hence almost entirely unionised at physiological pH and doesn't exist an the active, ionised form
- has its clinical effect by causing membrane expansion (this is a commonly repeated MCQ)
Minimum Effective Concentration (Cm)
- The minimum concentration of a local anaesthetic at the nerve fibre that will block conduction of the action potential
- Larger dia fibre → higher Cm
- ↓Cm by:
- ↑pH (LAs don't work well in inflammed, acidic tissue)
- ↑ frequency of stimulation
- Cm is different for different LAs, reflecting diff potency
- Cm motor fibre ~2 x Cm sensory fibre
- Minimum 2-3 Nodes of Ranvier need to be exposed to Cm to cause conduction block, otherwise AP may jump across the blocked section
Differential Conduction Block
- Preganglionic sympathetic B fibres blocked first then
- C, Adelta fibres (pain and temp) then
- large A fibres (touch, proprio, motor)
Toxicity
Types of Toxicity
A Systemic
- Relates to peak plasma conc, which will relate to
- dose
- rate of vasc absorption (protein binding of the LA, site of injection, use of v/c)
- CNS toxicity
- CVS toxicity
- CC:CNS ratio
B Hypersensitivity
- Due to para-aminobenzoic acid (PABA), a metabolite of ester LAs
- Due to methyl paraben (preservative used in LAs)
C Local Neurotoxicity
- Central neuraxial block
- Transient Radicular Irritation
- severe pain lower back, bum, post thighs
- onset < 24hr after recovery
- full recovery < 1wk
- Cauda Equina Syndrome
- sensory anaesthesia
- bowel and bladder sphincter dys function
- paraplegia
- associated with the use of small gauge catheters
- Peripheral nerve injury
- Intraneural injection of LA during nerve block
- Mechanisms
- Direct neurotoxicity effect (all LAs are neurotoxic in high concs)
- Pressure effect causing ischaemia
- Needle trauma
D Agent Specific
- Prilocaine
- metHb
- Cocaine
- vasoconstriction
- cardiac ischaemia
- CVA
CNS Toxicity
- Initially circumoral and tongue tingling/numbness (highly vascular)
- Cross BBB, initially block inhibitory interneurones→
- Restlessness
- Vertigo
- Tinnitus
- Difficulty focusing
- Slurred speech
- Skeletal mm twitching
- Tonic-clonic seizures
- Then all CNS neurones depressed
- ↓BP
- Apnoea
- Coma
Dose-Dependent Systemic Effects of Lignocaine
| Plasma Concentration (mcg/mL) | Effect |
|---|---|
| <1–5 | Analgesia |
| 5–10 | Numbness of tongue |
| 10–15 | Seizures Unconsciousness |
| 15–25 | Coma Respiratory arrest |
| > 25 | Cardiovascular depression |
CVS Toxicity
- Vasodilation of arterioles
- direct action on vasc smooth mm
- inhibition of sympathetic output
- Myocardial depression. Block VGSC → ↓[Na]i → ↓ [Ca]i
- Conduction block and bradycardia, ↑PR ↑QRS duration
CC:CNS Ratio
- Ratio of the dose that causes Cardiovascular Collapse to the dose that causes CNS toxicity
- The higher the CC:CNS ratio, the "safer" the LA as the CNS toxicity symptoms give earlier warning of systemic toxicity occuring (similar concept to the therapeutic ratio)
- Allows time for
- cessation of administration of LA
- institution of treatment before CVS collapse occurs
| CC:CNS Ratio | |
|---|---|
| Lignocaine | 7 |
| Bupivacaine | 3 |
| Ropivacaine | 5 |
Why is bupivacaine toxicity worse than ropivacaine toxicity?
- Bupivacaine has a lower CC:CNS ratio, so gives less warning of impending cardiovascular collapse
- Bupivacaine is a racemic mixture that contains 50% R-bupivacaine (dextro-bupivacaine), while ropivacaine is a pure S-enantiomer. R-enantiomers have a higher propensity to bind cardiac VGSCs compared to S-enantiomers.
- Bupivacaine has a longer dwell time (slower to dissociate from VGSC), so CVS collapse is more refractory to treatment than CVS collapse due to ropivacaine or lignocaine
Maximum Doses
- Systemic toxicity is associated with the plasma concentration, not the dose administered, per se
- Peak plasma conc relates to
- dose given
- rate of vasc absorption (protein binding of the LA, site of injection, use of v/c)
- Thus for a particular LA, the maximum safe dose actually varies depending on the site of injection
- For simplicity, most references quote a single value
| Local Anaesthetic | Max Dose (mg/kg) | Toxic Plasma Conc (ug/ml) |
|---|---|---|
| Lignocaine | 4 | > 5 |
| Lignocaine w/ adr | 7 | |
| Bupivacaine | 2 | > 1.5 |
| Levo-bupivacaine | 2 | |
| Ropivacaine | 3 | > 4 |
Treatment of Systemic Toxicity
- Standard ALS algorithm
- Intralipid 20%
- Intravenous lipid emulsion
- Acts as a 'lipid sink' - binding to lipid soluble LA and effectively removing it from circulation
- May have other mechanisms of action
- Direct prevention of VGSC inhibition
- Provide an alternative energy substrate, offsetting LA inhibition of fatty acid metabolism
- Dose:
- 1.5ml/kg over 1 min
- Followed by infusion of 0.25ml/kg/min
- Maximum recommended dose of 10ml/kg in first 30 mins
Regional
Neuraxial Block
Sites of Action
Intrathecal Injection
- Local anaesthetic can freely diffuse in the CSF
- Has direct access to blocking
- nerve roots
- spinal cord
Epidural Injection
- Local anaesthetic can block nerve transmission at various sites
- Extradural nerve roots
- LA can spread laterally out of the epidural space, through the intervertebral foramina to block nerve roots as they exit the dural cuffs
- Probably the primary mechanism of action
- Intrathecal nerve roots and spinal cord
- LA can diffuse through the dura into the CSF to reach these targets
Nerve Types Blocked
- Sympathetic
- Sensory
- Motor
- (Parasympathetic)
- Only the sacral component of the craniosacral parasympathetic outflow will be blocked (unless you get brainstem anaesthesia!)
- We don't usually consider this, as the sympathetic block has much more clinical relevance
Determinants of Block Height
NB Both intrathecal and epidural blocks result in a segmental block, ie have both an upper and a lower limit, but because clinical issues arise much more commonly from the upper limit being too low than from the lower limit being too high, we tend to ignore the lower limit and only talk about the upper limit "block height"
Intrathecal Block
Drug/Technique Factors
- Baricity and patient position
- This is probably the most important factor.
- Hyperbaric solution. When patient is supine, the solution will spread from the lumbar lordosis downward with gravity, cephalad towards thoracic kyphosis and caudad towards sacrum
- Isobaric solution. Spread is not influenced by patient position (NB plain 0.5% bupivacaine is actually slightly hypobaric, so tends to float up)
- Drug dose, concentration and volume
- The higher the mg dosage, the greater the spread and block height
- Concentration and volume are not important per se, ie giving 8ml of 0.2% rop would result in the same block height as 1.6ml of 1% rop
- This is because the drug is injected directly into a liquid, the CSF, and the drug can quickly diffuse around evenly
- Think about dropping a drop of food colouring into a glass of water - the colour will spread through the water pretty quickly. If you took another drop of water, then first diluted that with a small amount of water (analogous to a lower conc, higher vol, but same "dose" of colour) then dropped that into another glass of water, the spread of colour will be very similar to the first glass
- Level of injection
- Hyperbaric solution. The level of injection does not influence the block height as the effect of gravity and patient positioning is much more important
- Isobaric solution. The block height will be higher if injection is made at a higher level, ie L2/3 vs L4/5
Patient Factors
- Height and obesity
- May play a role (taller pt has larger intrathecal volume, therefore same dose results in lower block height while obese patient has compression of thecal sac resulting in reduced intrathecal volume and greater spread), but these effects are not consistently reproducible
- Pregnancy
- Increased sensitivity to the effects of LAs and thecal sac compression → need reduced dose for same block height
Epidural Block
Drug/Technique Factors
- Patient position
- While logically, the injected LA should spread following gravity, when studied, altering patient position has not been reliably shown to alter the direction of spread and block height
- Drug dose, concentration and volume
- In contrast to intrathecal injection, injection into the epidural space is injecting into a "potential" space - ignoring the epidural fat, blood vx, etc - so that the more volume that is injected, the more the space is spread open and the further up and down the vertebral column the LA will spread
- Volume is the most important determinant of block height
- From studies, roughly 2/3 of the injectate will spread cephalad and 1/3 will spread caudad
- Using a higher concentration will result in a more dense block, but won't increase the block height as much as increasing the volume given
- Level of injection
- An epidural block produces a more obviously segmental block with a discernible upper and lower limit
- The block will be centred around the level of insertion, so a thoracic epidural will produce a higher block than a lumbar one
Patient Factors
- Height and obesity
- Again, may play a role similar to with intrathecal injection, with a taller person having a longer epidural space and an obese person having a squashed epidural space, however effects not reliably reproducible
- Pregnancy
- Increased sensitivity to the effects of LAs and epidural space compression → need reduced dose for same block height
| Factor | Intrathecal Block | Epidural Block |
|---|---|---|
| Drug/Technique Factors | ||
| Baricity/Positioning | +++ | + |
| mg dose | +++ | |
| Concentration | + | |
| Volume | +++ | |
| Patient Factors | ||
| Height | (+) | (+) |
| Obesity | (+) | (+) |
| Pregnancy | + | + |
Adjuvant Drugs
Drugs that affect LA pharmacokinetics
- Adrenaline
- vasoconstriction delays vascular removal and prolongs duration of lignocaine
- Bicarbonate
- ↑pH → ↑unionised fraction of LA → faster onset
Drugs that affect LA pharmacodynamics (synergistic effect)
Peripheral Blocks- Opioids
- Peripheral opioid receptors have been identified
- Studies are equivocal about whether adding opioid to a peripheral nerve block enhances duration or quality
- Dexamethasone
- Has been shown to prolong duration of peripheral nerve block
- Mechanism not well understood, but thought to be a steroid receptor dependent, locally mediated effect
- Magnesium
- ?mech
- Dexmedetomidine
- ?mech
- Fentanyl
- ↑block density and prolongs duration of surgical anaesthesia and analgesia
- Synergistic effect with LAs, via action on opioid mu receptors
- Morphine
- Has slow onset of action due to relatively poor lipid solubility (about 1hr) so is useful for post-op analgesia but does not contribute much to intra-op anaesthesia
- Risk of delayed respiratory depression (18-24hrs post administration) due to slow removal from CSF by vascular uptake and circulation in CSF up to brainstem and depressing the respiratory centre
- Other potential side effects
- pruritis (40-60%)
- nausea
- urinary retention (males)
- reactivation of latent herpes simplex in facial nerve
- Clonidine
- acts on a1 receptors on descending inhibitory pain pathways
- Adrenaline
- acts on a1 receptors on descending inhibitory pain pathways
EMLA
Eutectic Mixture
- A mixture of substances (in fixed proportions) that has a single melting point that is lower than the melting point of either constituent alone
5% EMLA (Eutectic Mixture of Local Anaesthetics)
- 2.5% lignocaine, 2.5% prilocaine
- NaOH to pH 9.6
- Purified water
- Dose 1-2g/10cm2
- Onset 5 mins
- Max effect 45 mins
- EMLA has melting point of 16C
- It is a LA droplet in water emulsion
- It has a conc of 100% within the emulsion droplet, as it is pure local anaesthetic in liquid form
Transdermal Application
- Onset, efficacy, duration affected by:
- Skin blood flow
- Epidermal/dermal thickness, skin pathology
- Duration of application
Adverse Effects
- Metabolite of prilocaine (o-toluidine) can cause methaemoglobinaemia, esp infants < 3 mo (immature reductase pathway)
- Vasoconstriction, making cannulation more difficult
Why is EMLA an effective skin topical local anaesthetic, while 5% lignocaine is not?
- EMLA is a eutectic mixture of 2.5% prilocaine and 2.5% lignocaine, which has a melting point of 16C, ie the local anaesthetic mixture is a liquid at room temperature with a 100% concentration within the droplets
- It is presented as a LA droplet in water emulsion and at the droplet/skin interface, there is an effective LA concentration of 80% - I don't know why it's not 100%, but this is what the textbooks say, maybe it's taking into account dilution by the emulsifying agent?
- Thus the rate of diffusion through the skin is much higher than for 5% lignocaine, in accordance to Fick’s Law of diffusion, making it more effective as a skin topical anaesthetic