Definitions
Shunt (True shunt)
- Venous blood which enters the arterial system without passing through the gas exchanging parts of the lung
Venous Admixture
- The amount of mixed venous blood that would have to be added to pulmonary end-capillary blood to produce the observed drop in arterial pO2 from the pO2 in end-capillary blood
Physiological Dead Space
- The part of the tidal volume that does not participate in gas exchange.
- Anatomical + alveolar DS
Anatomical Dead Space
- The volume in the conducting airways
Alveolar Dead Space
- The part of the tidal volume that passes through the conducting airways to mix with gas at the alveolar level, but which does not participate in gas exchange
- Alveoli that are ventilated but not perfused.
- West zone 1
Apparatus Dead Space
- The part of the breathing circuit where to-and-fro movement of gas occurs
V/Q Mismatch
- Any deviation in the ventilation to perfusion ratio away from the ideal V/Q of 1
- Encompasses a spectrum from V/Q 0 (shunt) to V/Q ∞ (alveolar dead space)
West Zones
West Zone 1
PA > Pa > Pv
West Zone 2
Pa > PA > Pv
West Zone 3
Pa > Pv > PA
West Zone 4
Region of reduced blood flow at the base of the lungs. This is due to the weight of the lung compressing the base → smaller lung volume → ↓ radial traction holding extra-alveolar vessels open
Hypoxaemia
- Abnormally low pO2 in arterial blood (pO2 < 60mmHg)
Hypoxia
- Oxygen delivery to the tissues that is inadequate to meet its metabolic demands
Functional Residual Capacity
- The equilibrium point between the elastic recoil of the lungs and the elastic recoil of the chest wall
- The volume of gas remaining in the lungs at the end of normal expiration
- FRC = RV + ERV
Closing Capacity
- The lung volume below which the small airways start to collapse
Hypoxic Pulmonary Vasoconstriction
- HPV is a homeostatic mechanism that aims to maintain optimal V/Q matching by diverting blood away from poorly ventilated areas of lung
Oxygen Flux Equation
| O2 flux | = Chemical O2 delivery + Dissolved O2 delivery |
| = CO x [Hb] x SaO2 x k + CO x paO2 x 0.003 | |
| = 50dL/min x 15g/dL x 0.99 x 1.34ml O2/g + 50dL/min x 100mmHg x 0.003ml O2/mmHg/dL | |
| = 1000ml O2 / min |
Starling Resistor
- A system in which the driving pressure for flow is equal to
- (upstream P - extraluminal P)
- In contrast to most situations where driving pressure equals
- (upstream P - downstream P)
Laplace's Law
- P = 4 T / r
- P - distending pressure within the lumen of a sphere
- T - surface tension along the surface of the sphere
- r - radius of the sphere
Total Minute Ventilation
- The total volume of gas leaving the lungs each minute
Alveolar Minute Ventilation
- The part of the minute ventilation that reaches the alveolar gas compartment where gas exchange occurs
Diffusion Limited Gas Transfer
- Amount of gas transferred from alveolus to capillary blood, per unit time, is limited by the diffusion properties of the blood-gas barrier, and not by the amount of blood flow
- Partial pressures in alveolar gas and blood DO NOT equilibrate before the blood exits the pulmonary capillary
Perfusion Limited Gas Transfer
- Amount of gas transferred from alveolus to capillary blood, per unit time, is limited by the amount of blood flow, and not by the diffusion properties of the blood-gas barrier
- Partial pressures in alveolar gas and blood equilibrate before the blood exits the pulmonary capillary
Haldane Effect
- Describes the phenomenon whereby deoxygenation of haemoglobin increases the ability of blood to carry CO2
- O2 unloading assists with CO2 loading in blood
Bohr Effect
- Describes the phenomenon whereby increased CO2 in blood results in a reduced affinity of haemoglobin for O2, causing a right shift in the ODC
- CO2 loading assists with O2 unloading in blood
Compliance
- Change in volume per unit change in pressure
Specific Compliance
- Compliance / FRC
Hysteresis
- The phenomenon whereby the transmural pressure required to distend an elastic body, such as the lungs, is greater during expansion compared to during contraction, for a given volume
Time Constant
- The time constant is a concept that describes the time course of an exponential process
- One time constant is the time taken for an exponential process to be 63% complete
- After 3 time constants, an exponential process will be 95% complete
Alveolar Gas Equation
pAO2 = FiO2 ( pB - pH2O) - pCO2 / R + F
pAO2
- alveolar partial pressure O2
FiO2
- inspired concentration of O2
pH2O
- saturated vapour pressure of water at 37C
pCO2
- arterial partial pressure of
CO2
R - respiratory quotient (0.8 for a typical diet)
F - small correction factor (ignore this)
Functional anatomy
Airways
- Conducting zone
- Trachea
- Bronchi
- Bronchioles
- Terminal bronchioles
- Respiratory zone
- Respiratory bronchioles
- Alveolar ducts
- Alveolar sacs
- Gas movement in conducting airways by bulk flow
- Gas movement in resp zone mainly by diffusion due to large x-sectional area
Blood Vessels
- Pulmonary artery pressures 25/8mmHg (mean 15mmHg)
- Capillaries 10um dia
- Each rbc spends 3/4 sec in capillary network, allowing equilibration of O2 and CO2
- Additional blood system, the bronchial circulation, supplies conducting airways down to terminal bronchioles, blood carried away by pulmonary veins (contributes to shunt)
Muscles of Respiration
Muscles of Respiration
- Diaphragm
- Dome shaped muscle that inserts into the lower ribs
- External intercostal muscles
- Bucket handle movement
- Accessory muscles
- Scalene mm
- SCM
- Expiration
- Usually passive
- Active
- Abdominal mm
- Internal intercostal mm
Diaphragm and functions
- C- shaped structure of muscle and fibrous tissues
- Muscle fibres arise from peripheral attachment to form central tendon → crest of diaphrgam (thin but strong aponeurosis)
- 3 Openings: Aorta (T12), oesophagus (T10) and vena caval (T8)
- Front arises from xiphoid process, costal margins
- Laterally inserts to ribs 6-12
- Back attaches to T12 vertebral body, descents to L1/L2 on sides
- Innervation
- Efferent and central afferent: phrenic nerve (C3-5).
- Peripheral afferents from intercostal and subcostal nerves
- Blood supply
- Above from internal thoracic artery
- Below from inferior phrenic artery
- Drains into IVC and supra-renal veins
- Functions:
- Respiration
- Separates abdominal and thoracic contents
- Vomiting
- Preventing GORD
- Urination/Defecation (increase IAP)
Surfactant
Surfactant
- Alveoli 0.3mm dia
- High surface tension of liquid lining the alveoli tends to collapse them
- Surfactant dramatically reduces surface tension
- Laplace's Law: P = 4 x T / r (for a sphere)
- P - distending pressure within the lumen of a sphere
- T - surface tension along the surface of the sphere
- r - radius of the sphere
- Surfactant is mainly phospholipid (dipalmitoyl phosphatidylcholine DPPC), some protein and carbohydrate
- DPPC is amphipathic (charged choline head, hydrophobic palmitoyl tails)
- Produced by Type II alveolar epithelial cells
- DPPC forms a monolayer at the gas-fluid interface, with the hydrophilic heads towards the water and hydrophobic tails towards the gas
- When this occurs, their intermolecular repulsive forces counteract the attracting forces between the molecules of the surface liquid
Roles of surfactant
- ↑ Lung compliance
- ↑ Stability of alveoli (without surfactant, the higher surface tension in smaller alveoli would result in a higher luminal pressure → gas movt from smaller to larger alveoli)
- Keep alveoli dry (high surface tension → ↓ hydrostatic pressure in tissue around capillaries and encourages fluid exudation). Surfactant reduces this effect
- Surfactant is the primary cause of lung hysteresis (see section on compliance)
Functions of the Nose
- Part of the conducting airways
- Humidification
- Warming
- Filtration
- Olfaction
- Resonator in speech
- Provides 1-2cmH2O PEEP
What is the mechanism for warming?
- Heat transfer from the vascular cavernous plexuses, arranged longitudinally in the nose
Where does humidification occur in the nose?
- Turbinates provide a large mucosal surface area and turbulent flow around the turbinates results in maximal contact of inspired gas with the mucosal surface, maximising the vaporization of water
- The water comes mainly from the exudation from mucous surfaces, partly from mucus and serous glands and goblet cells
- Inspired gas starts to be humidified as it passes through the nose and is fully saturated by the time it reaches the carina (absolute humidity 44mg/L, 100% relative humidity at 37C)
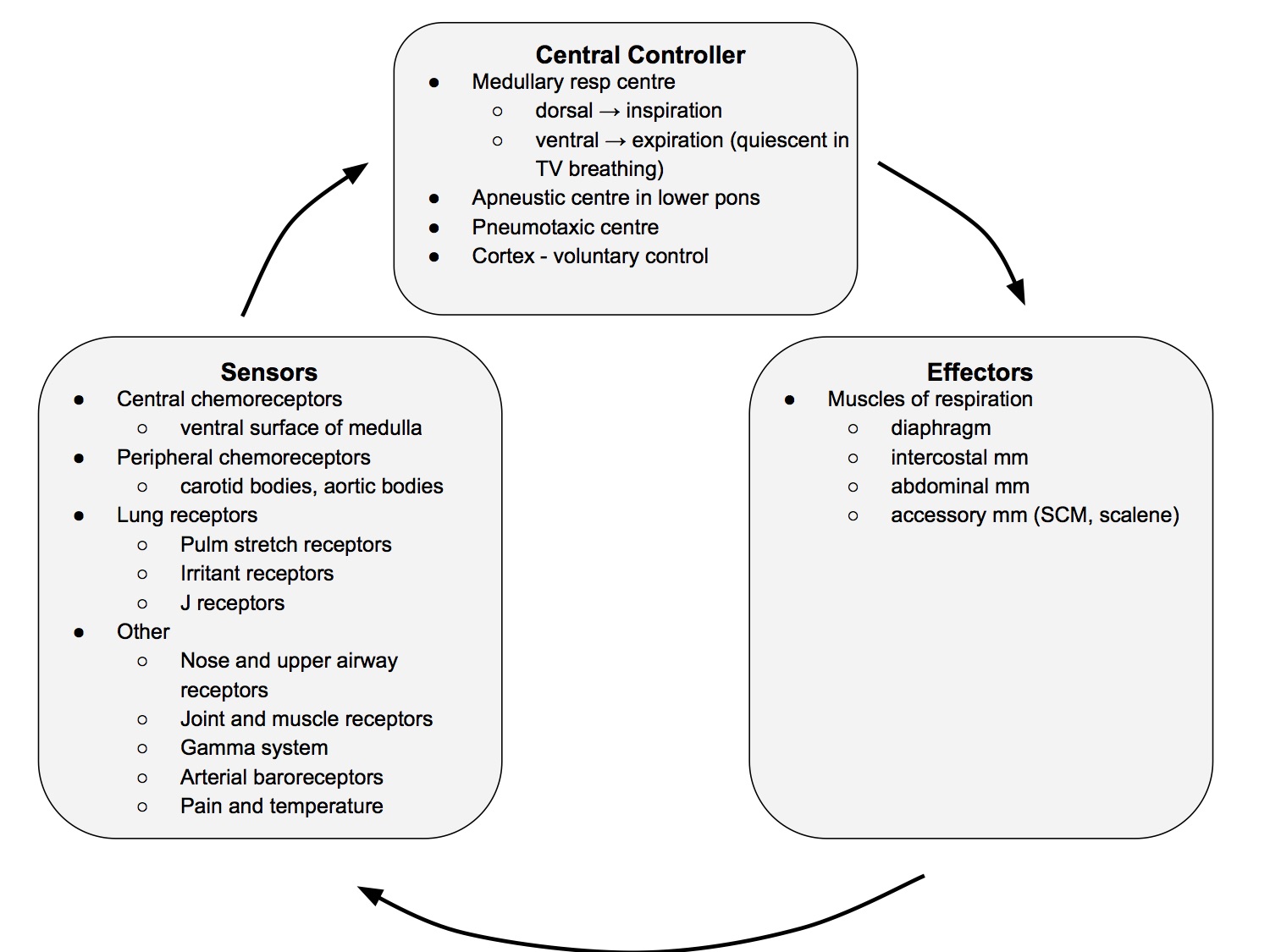
Control of Respiration
Schematic
Lung Volumes & Capacities
Spirometry
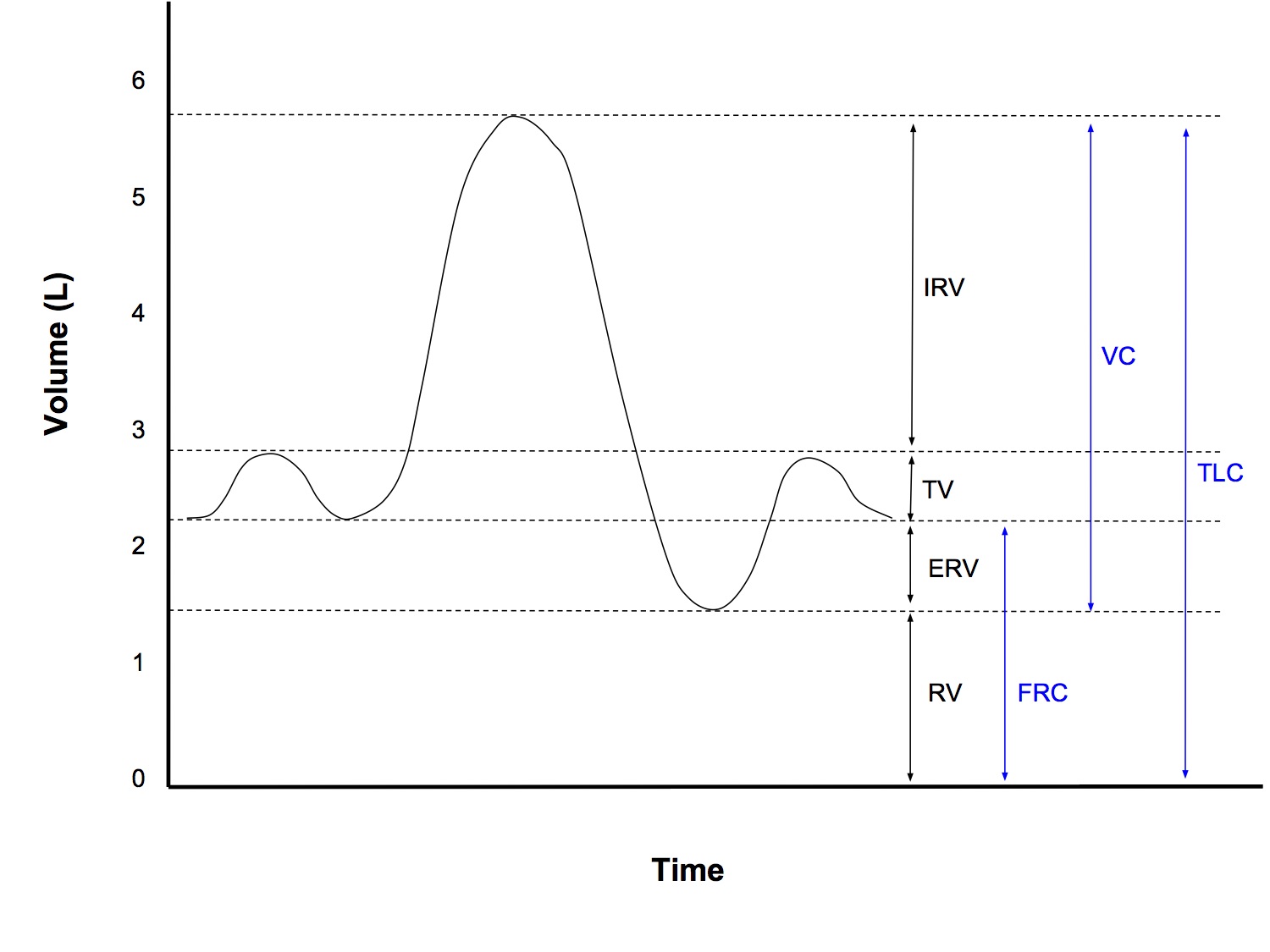
Volumes
- Smallest named subdivisions of lung volumes
- Inspiratory reserve volume
- from end of normal inspiration to maximal inspiration
- Tidal volume
- from end of normal expiration to end of normal inspiration
- Expiratory reserve volume
- from end of normal expiration to maximal expiration
- Expiratory reserve volume
- from end of normal expiration to maximal expiration
- Residual volume
- the volume left in the lungs at maximal expiration
- due to closure of small airways from reduced radial traction holding airways open and gas trapping
Capacities
- The sum of 2 or more volumes
- Functional reserve capacity
- the volume left in the lungs at the end of normal expiration
- Vital capacity
- from maximal inspiration to maximal expiration
- Total lung capacity
- the volume in the lungs at maximal inspiration
Normal Values
- RV 20ml/kg
- FRC 30ml/kg
- TV 7ml/kg
- TLC 80ml/kg
- All the other volumes/capacities are less useful to remember and can be derived from the above values
Measurement of Lung Volumes
- Spirometry can be used to measure all volumes/capacities, except for those that include the residual volume
- FRC, which includes the RV, can be measured by
- Helium dilution technique - measures only gas that communicates with the mouth
- Body plethysmography - uses double application of Boyle's Law. Measures total volume, including trapped gas
See West for full explanations
Functional Residual Capacity
Definition
- The equilibrium point between the elastic recoil of the lungs and the elastic recoil of the chest wall
- The volume of gas remaining in the lungs at the end of normal expiration
- FRC = RV + ERV
Normal Value
- 30ml/kg supine
- 40ml/kg erect
Factors Affecting FRC
FRC increased with
- Height (taller → ↑ FRC)
- Position (FRC greater by 30% erect vs supine)
- ↑ Lung elastic recoil (emphysema)
FRC decreased with
- Obesity
- Muscle paralysis (remove muscle tone of diaphragm)
- ↑ Lung elastic recoil (pulm fibrosis)
- Pregnancy
- Anaesthesia
FRC vs Closing Capacity
- FRC does not change with age (both lung and chest wall elastic recoil ↓ with age)
- However, CC ↑ with age (less radial traction holding small airways open)
- CC > FRC @ 44yo, supine
- CC > FRC @ 66yo, erect
Functions of FRC
- O2 store
- Buffer to maintain steady arterial pO2
- Prevent atelectasis
- Minimise work of breathing
- Minimise PVR
- Keep airway resistance low
Mechanics of Breathing
Compliance
- Change in volume per unit change in pressure
- Typical values:
- Lung compliance 200ml/cmH2O
- Chest wall compliance 200ml/cmH20
- Total respiratory system compliance (lung and chest wall together) 100ml/cmH20
- 1 / Ctotal resp system = 1 / Clungs + 1 / Cchest wall
- Specific lung compliance 0.05cmH2O-1
- Specific compliance is compliance / FRC
- This allows comparison between different sized lungs and specific compliance is the same in neonates and adults
- NB The above figures are quoted in Brandis, but don't make sense to me. In an adult, if lung compliance is 200ml/cmH2O and FRC is 2000ml, then specific compliance should be 200/2000 = 0.1cmH2O-1! I wouldn't worry too much about it. If asked in a viva, just mention that there is a discrepancy between values quoted in texts
- The slope of the lung compliance curve is not uniform, so by convention, lung compliance is calculated from the slope of the line joining the points at FRC and FRC + 1L on the expiratory limb of the compliance curve
Factors Affecting Compliance
- Lung size (absolute)
- Larger lungs, eg adult vs neonate → ↑ compliance (specific compliance remains constant)
- Lung volume (relative)
- The compliance curve is steepest in the mid range (compliance is greatest around FRC, while at the extremes (high and low lung volumes), compliance is reduced
- Pulmonary blood volume
- Blood vx contribute to stiffness of the lungs
- Congestion → ↓ Compliance
- Age
- Small ↑ compliance with ↑ age due to changes in elastin and collagen (elasticity mainly determined by surface tension)
- Bronchial smooth m tone
- ↑ airway resistance → ↓ dynamic, but not static, compliance
Compliance Curve
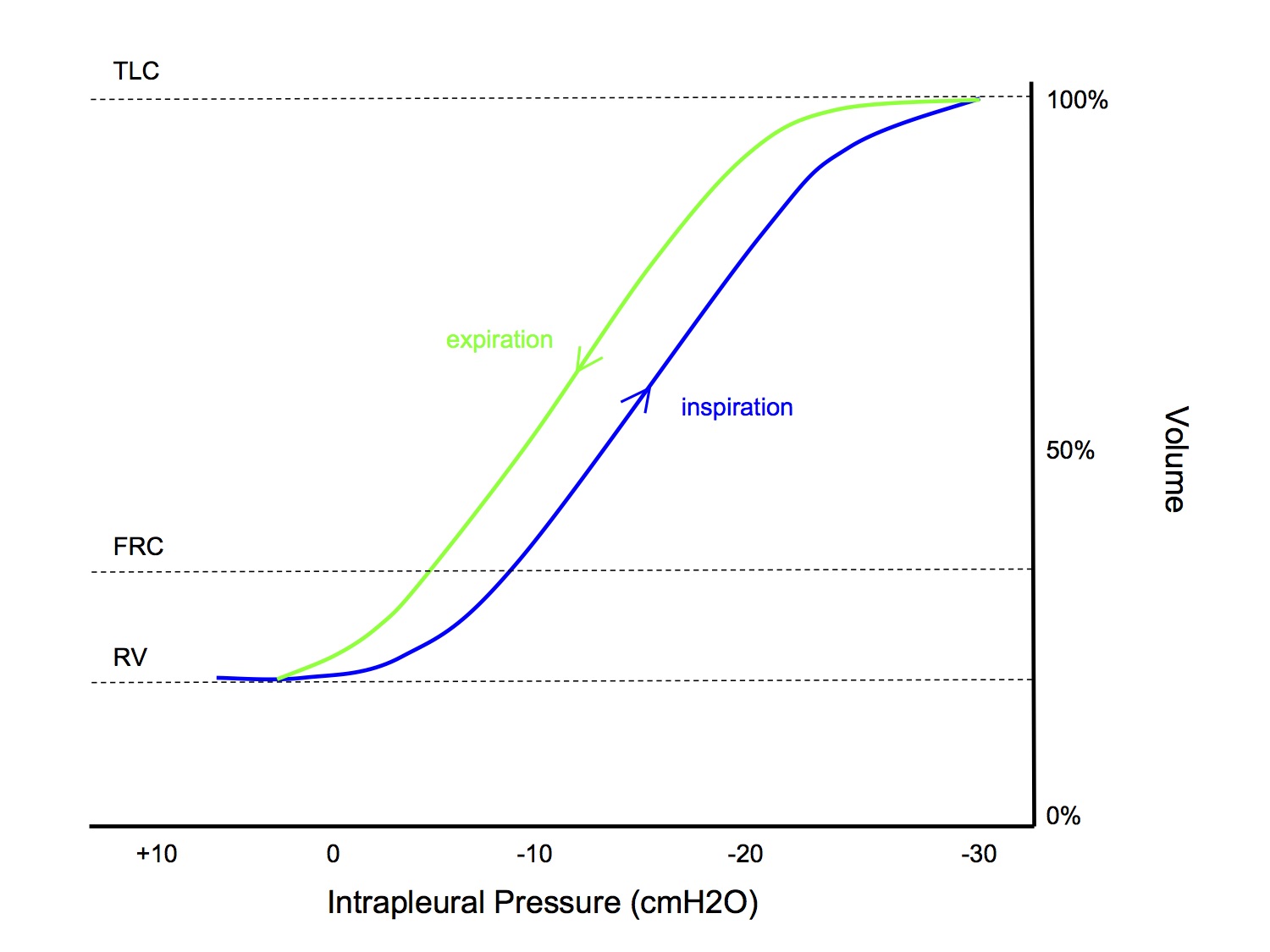
- Compliance curve is sigmoid shaped
- At low volumes, compliance is less because of Laplace's law - higher distending pressures are required to overcome surface tension at smaller volumes (like how a balloon is initially harder to blow up, then becomes easier as the volume increases)
- At high volumes, compliance is less because of increased stretch on elastic tissue
- The shape of the curve is different during inspiration and expiration (higher pressures during inspiration at the same volume during expiration), this is called hysteresis (see next section, Time Dependence of Pulmonary Elastic Behaviour)
- The concept of compliance can be applied to the lungs as a whole, and also to specific parts of the lung, eg apex vs base
Common SAQ questions
- What are the effects of decreasing FRC by 1L?
- What regional differences are there between the apex and the base of the lungs?
- Both of those are very broad questions, that require you to address many aspects of lung physiology: compliance, airway resistance, pulm vascular resistance, V/Q matching... but in terms of just addressing the compliance aspect, the same knowledge can be used to answer both questions
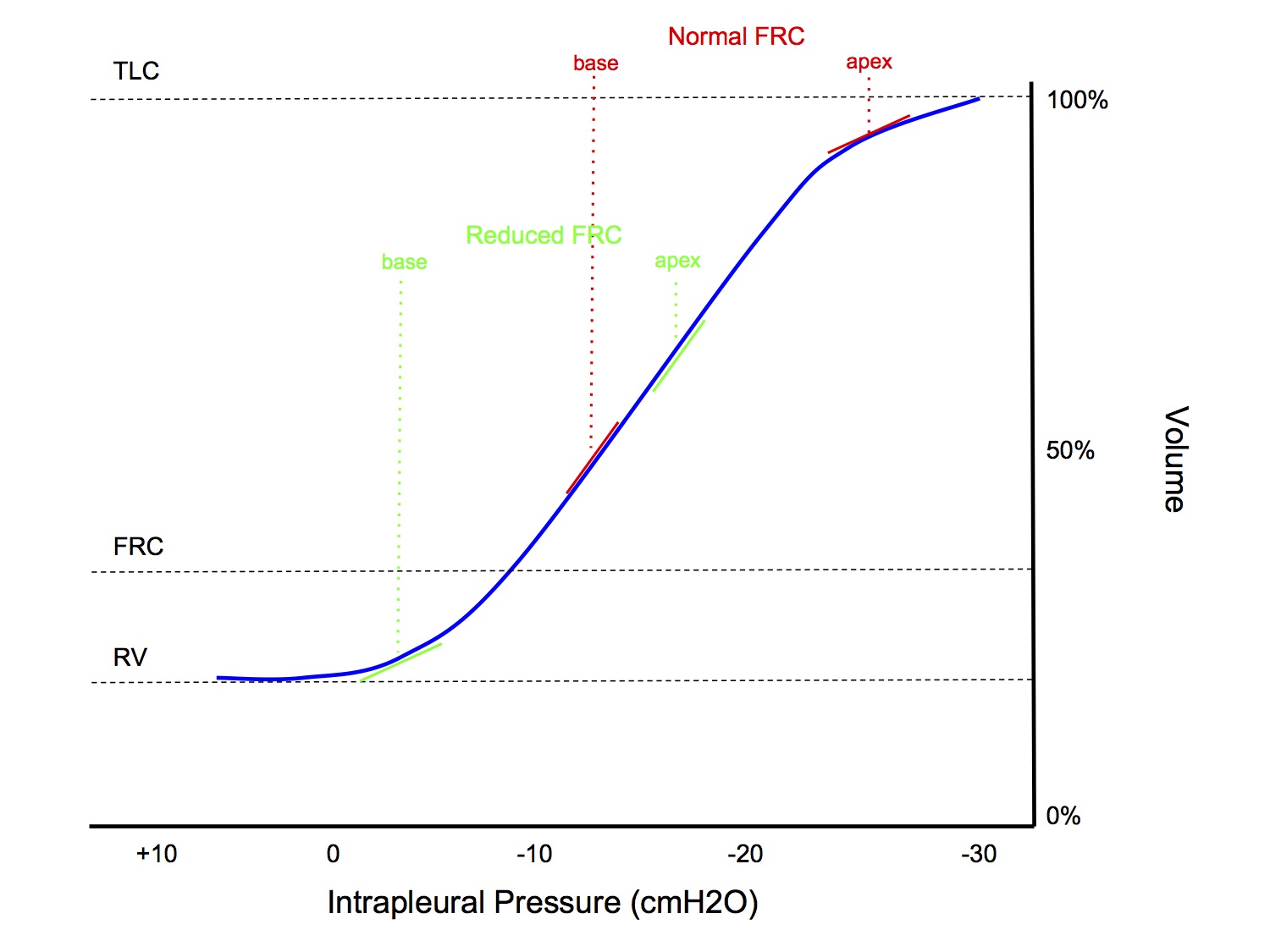
- In the usual situation (with normal FRC), the base of the lung operates on the steep middle part of the compliance curve and receives more ventilation than the apex, which operates on the flatter upper part of the compliance curve (see section Regional Differences → Apex vs Base)
- If FRC is reduced, then the apex of the lungs shifts down to the steep, middle part of the curve and receives more ventilation than the base which is now on the flatter, lower part of the curve, ie a reversal of the normal situation
Time Dependence of Pulmonary Elastic Behaviour
Hysteresis
- The phenomenon whereby the transmural pressure required to distend an elastic body, such as the lungs, is greater during expansion compared to during contraction, for a given volume
- It is caused by time dependent behaviours of the lung
Static vs Dynamic Compliance
- Static compliance is measured by getting the subject to hold their breath at various lung volumes, before taking pressure measurements - to allow time for equilibration of time dependence processes to occur - while dynamic compliance is measured during normal, rhythmic breathing (see next section Measurement of Compliance)
- Static compliance is always greater than dynamic compliance and this is also due to time dependent behaviours of the lung
Causes of Time Dependent Behaviour
- Changes in surfactant activity
- During expiration (contraction), the surfactant molecules are crowded together, thus having a greater effect in reducing surface tension
- This is probably the most important cause of hysteresis in the lung
- Stress relaxation
- An inherent property of elastic bodies
- If a spring is pulled out to a fixed increase in its length, the resultant tension is maximal at first then declines exponentially to a constant value
- This is a viscoelastic property that is displayed by collagen in lung tissue
- Contributes to difference in static and dynamic compliance
- Recruitment of alveoli
- Recruitment of collapsed alveoli during inspiration results in greater volumes for lower pressure during expiration
- Redistribution of gas (pendelluft)
- During a short inflation, gas preferentially goes to fast alveoli (with lower compliance, hence greater transmural pressure)
- Slow, sustained inflation, permits greater gas distribution to slow alveoli, in accordance to their compliance (hence lower transmural pressure)
- Extreme differences between fast and slow alveoli only occur in disease states, so gas redistribution is not an important factor in the healthy lung
- Time constants are used to describe exponential filling and emptying of a lung unit. 1 time constant = time to achieve 63% of maximal inflation or deflation of a lung unit
Importance of Factors
- Different texts disagree on which factors contribute to causing hysteresis and the difference between static/dynamic compliance (Nunn differs slightly from Power and Kam)
- Below is my interpretation of what is important. I don't know if it is correct, but don't worry about it, if you are getting asked on this in a viva, I think you are already doing very well!
| Factor | Hysteresis | Static/Dynamic Compliance Difference |
| Change in surfactant activity | Yes (most imp) | |
| Stress relaxation | Yes | Yes |
| Recruitment | Yes | Yes |
| Pendelluft | Yes (disease states only) |
Measurement of Compliance
- To measure compliance, we need to measure lung volumes and transmural pressures
- Which transmural pressure we use will depend on what system we want to measure compliance for
- Lung compliance: (alveolar - intrapleural) pressure
- Chest wall compliance: (intrapleural - ambient) pressure
- Total resp system compliance: (alveolar - ambient) pressure
- Lung volume can be measured by
- Spirometer
- Pneumotachograph
- Body plethysmography
- Pressures measured by
- Intrapleural - oesophageal manometer, inserted 32-35cm past the nares
- Alveolar - mouth pressure when no gas flow
Static Compliance
- At FRC, the subject relaxes against a closed airway and pressure readings are taken
- A known volume of air is inhaled then the subject relaxes against a closed airway and pressure readings are taken again
- This procedure can be repeated to plot points on the compliance curve and can be done during expiration to obtain the compliance curve during expiration (hysteresis)
Dynamic Compliance
- Measurements of volume and pressure are taken during normal, rhythmic breathing without any pauses
- The two points used to calculate compliance are at end-expiration and end-inspiration - the two points of no gas flow during the respiratory cycle. This is so that pressure measured at the mouth can be assumed to equal alveolar pressure
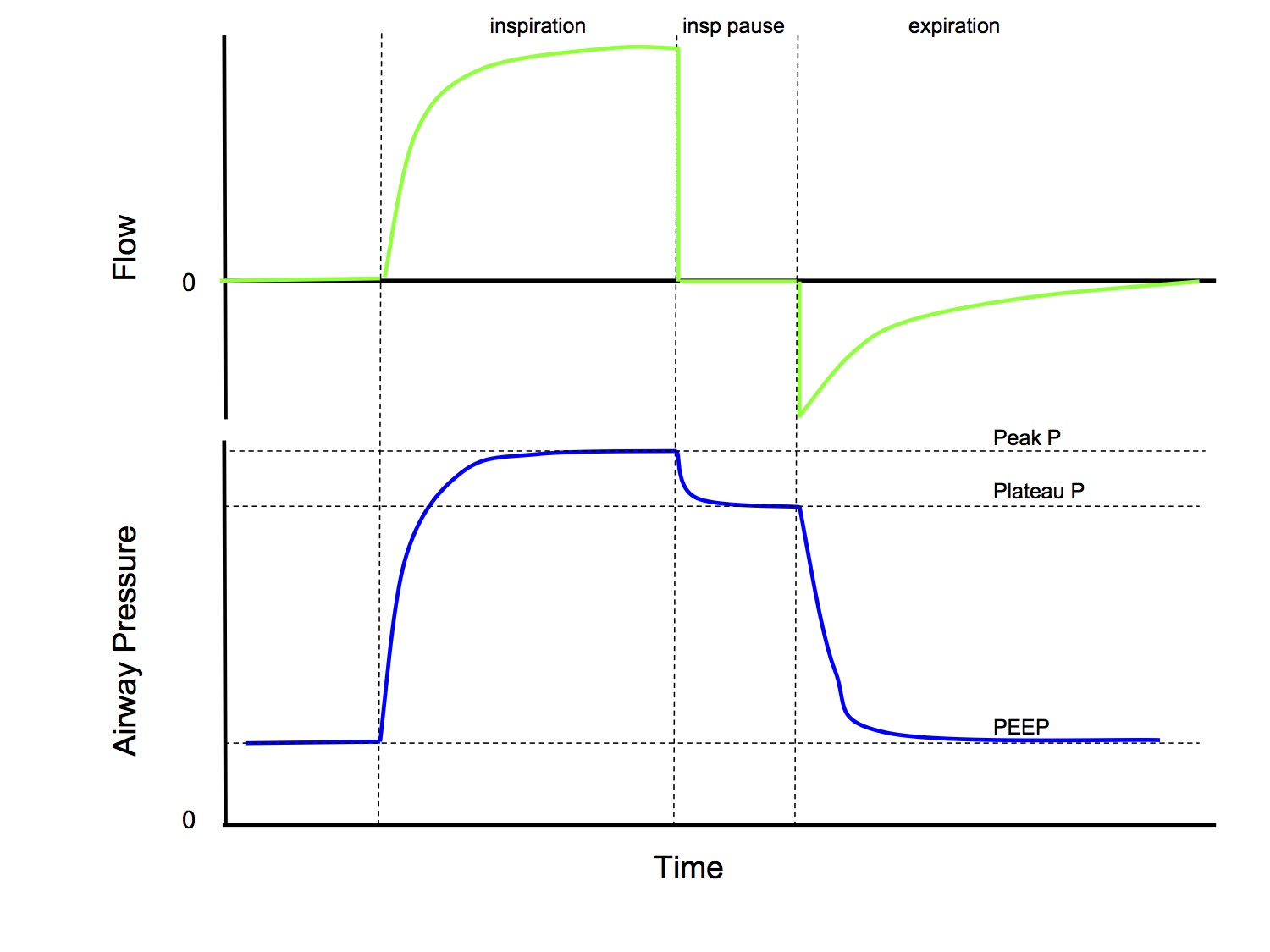
Can compliance be measured in an intubated and ventilated patient?
- Yes!
- If you simply calculate tidal volume / (peak inspiratory P - PEEP), this will calculate dynamic compliance
- If you use a volume control mode of ventilation and add an inspiratory pause, then you can calculate tidal volume / (plateau P - PEEP), which will calculate static compliance
- Note that both the above calculations calculate total respiratory system compliance and not lung compliance
Ventilation & Diffusion
Ventilation
Total minute ventilation
- The total volume of gas leaving the lungs each minute
- Vtotal = Valveolar + Vdeadspace
- 500 x 15 = 7,500 ml/min
Alveolar minute ventilation
- The part of the minute ventilation that reaches the alveolar gas compartment where gas exchange occurs
- Valveolar = Vtotal - Vdeadspace
- (500 - 150) x 15 = 5,250 ml/min
Minute Ventilation & paCO2
- All expired CO2 comes from alveolar gas
- VA = k . VCO2 / paCO2
- k - a constant
- VA - alveolar minute ventilation
- VCO2 - rate of CO2 production
- paCO2 - arterial partial pressure CO2
- paCO2 is inversely proportional to alveolar minute ventilation
- Assuming that metabolic rate (and hence rate of CO2 production) is constant, if minute ventilation halves, from 5L/min to 2.5L/min, eg from narcosis, then paCO2 will double from 40mmHg to 80mmHg
Perfusion vs Diffusion Limited Gas Transfer
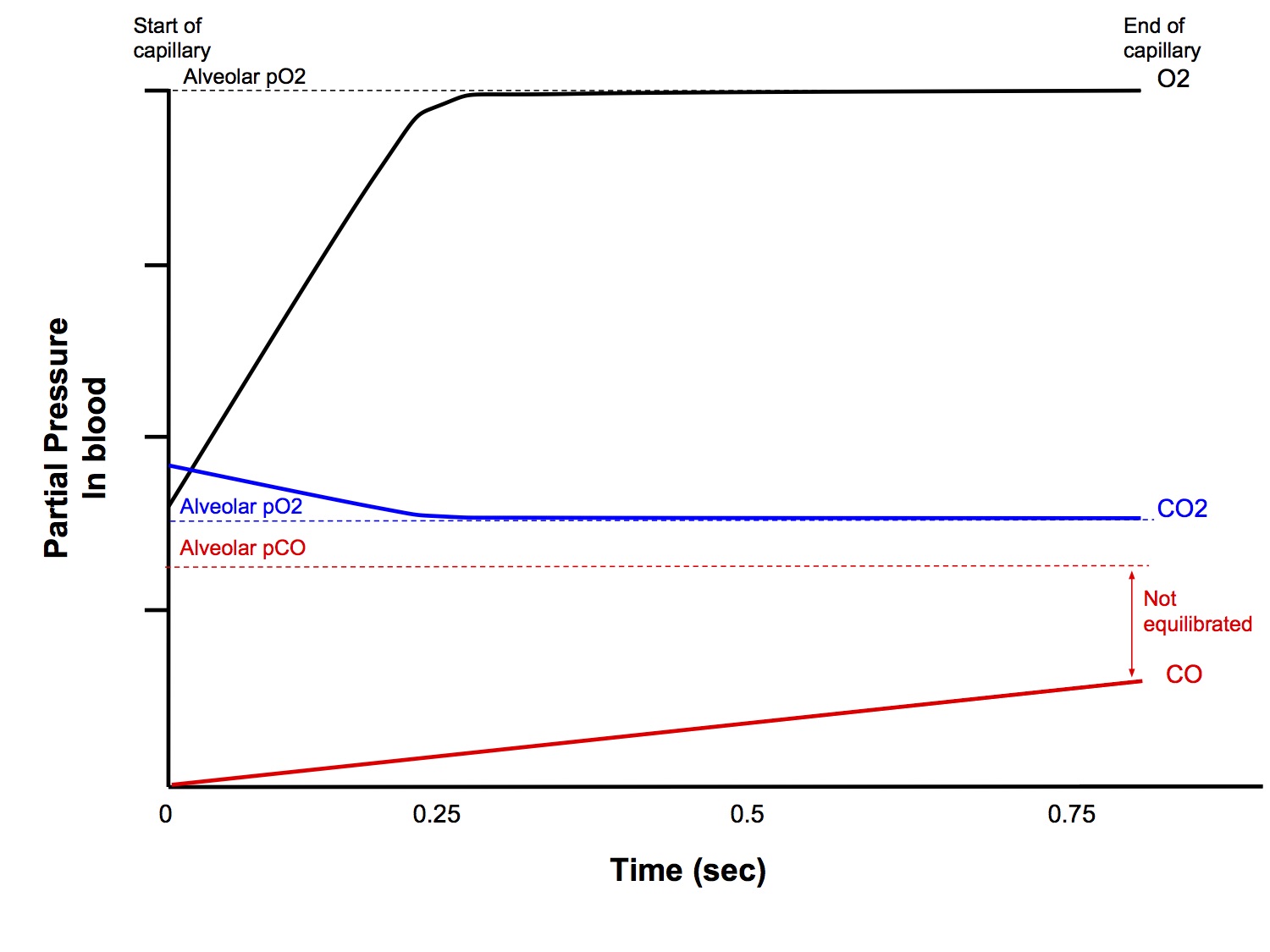
Diffusion Limited Gas Transfer
- Amount of gas transferred from alveolus to capillary blood, per unit time, is limited by the diffusion properties of the blood-gas barrier, and not by the amount of blood flow
- Partial pressures in alveolar gas and blood DO NOT equilibrate before the blood exits the pulmonary capillary
- eg CO, where CO rapidly reacts with Hb to form carboxyHb, thus maintaining a large partial pressure gradient
Perfusion Limited Gas Transfer
- Amount of gas transferred from alveolus to capillary blood, per unit time, is limited by the amount of blood flow, and not by the diffusion properties of the blood-gas barrier
- Partial pressures in alveolar gas and blood equilibrate before the blood exits the pulmonary capillary
- eg N2O, O2, CO2 (under normal circumstances)
Oxygen
- Under typical resting conditions, alveolar and capillary pO2 equilibrate in 0.25 secs, ie perfusion limited
- May become diffusion limited under abnormal circumstances
- Thickened blood-gas barrier
- Exercise (time in pulmonary capillaries can reduce to 0.25 secs
Carbon Dioxide
- Capillary and alveolar pCO2 equilibrate rapidly and so CO2 transfer is perfusion limited
Diffusion Capacity
- Transfer of CO in the lungs is diffusion limited, so it is the gas of choice for measuring diffusion capacity
- Ficks Law of diffusion
- Vgas = A / T . D . (P1 - P2)
- Vgas - rate of diffusion of a gas
- A - surface area available for diffusion
- T - thickness of the diffusion pathway (alveloar membrane thickness)
- D - diffusion coefficient
- P1 - P2 - partial pressure gradient over the diffusion pathway (alveolar to capillary blood gradient)
- Diffusion capacity of the lung (DL) combines the A, T and D into one variable, and thus the diffusion capacity for CO is
- DL = VCO / (P1 - P2) and since pCO in blood in negligible (as it rapidly combines with Hb to form carboxyHb) →
- DL = VCO/PACO
Single Breath Method for Measuring DL
- A single inspiration of dilute mixture of CO is made, then breath hold for 10 secs then expiration
- Infrared gas analyser measures inspired and expired CO concentrations
- Alveolar conc of CO is not constant during the breath hold but allowances can be made for that
- Normal value DL is 25 ml/min/mmHg
Alveolar Gas Equation
pAO2 = FiO2 ( pB - pH2O) - pCO2 / R + F
pAO2
- alveolar partial pressure O2
FiO2
- inspired concentration of O2
pH2O
- saturated vapour pressure of water at 37C
pCO2
- arterial partial pressure of
CO2
R - respiratory quotient (0.8 for a typical diet)
F - small correction factor (ignore this)
- You must be able to reproduce this equation without any hesistation!
- In vivas, it is commonly used to explain
- hypoxaemia due to low FiO2, hypoventilation
- the principle of pre-oxygenation and oxygen reservoirs
- the A - a gradient
Ventilation-Perfusion Relationships
Dead Space
Definitions
Physiological
- The part of the tidal volume that does not participate in gas exchange.
- Anatomical + alveolar DS
Anatomical
- The volume in the conducting airways
Alveolar
- The part of the tidal volume that passes through the conducting airways to mix with gas at the alveolar level, but which does not participate in gas exchange
- Alveoli that are ventilated but not perfused.
- West zone 1
Apparatus
- The part of the breathing circuit where to-and-fro movement of gas occurs
Factors Affecting Anatomical Dead Space
- Size of subject
- increases with body size
- 2.2ml/kg
- Age
- at infancy, anatomical dead space is higher for body weight (3.3ml/kg)
- Posture
- sitting 147mL, supine 101mL
- Position of neck and jaw
- Lung volume at the end of inspiration
- Anatomical dead space increases by 20mL for each L of lung volume
- Anatomical dead space is roughly 1/3 the tidal volume
- Drugs
- e.g. bronchodilator will increase dead space
Factors Affecting Alveolar Dead Space
- Alveolar dead space is West zone 1 where PA > Pa > Pv
- Any factors that ↑ PA or ↓ Pa will ↑ alveolar dead space
- IPPV
- high PEEP
- any cause of ↓ pulmonary perfusion, ie shock
Factors Affecting Apparatus Dead Space
- Do not confuse apparatus dead space with circuit volume! They are two very distinct concepts
- Apparatus dead space is the part of the breathing circuit where to-and-fro movement of gas occurs
- Circuit volume is the total volume within the circuit system
- In a circle circuit, the apparatus dead space extends from the Y-connector of the inspiratory and expiratory limbs, through the HME filter to the patient airway (ETT/LMA or mask), ie it is small
- However, because the circuit volume of a circle system is large (including the inspiratory and expiratory limbs, the CO2 absorber and the reservoir bag), changes made to the fresh gas composition take a long time to be reflected at the patient end. This is why gas induction using a circle circuit is slower than using a T-piece
Clinical Significance
- By definition, dead space does not participate in gas exchange, it is wasted ventilation (cannot contribute to oxygenation of the blood or elimination of CO2)
- Anatomical dead space
- Because anatomical dead space gas is the gas that is breathed out at the start of expiration and contains no CO2, on a capnograph expiration actually starts BEFORE the phase II expiratory upstroke (a common misconception is that expiration starts with the upstroke)
- Alveolar dead space
- Alveolar dead space is the cause of the arterial - end tidal CO2 difference (see section below)
- Alveolar dead space cannot cause hypoxaemia per se - by definition, it does not receive any perfusion, so how could it affect the gas composition in the blood!
- However, if total minute ventilation is fixed, eg in a paralysed, ventilated patient and there is ↑ in alveolar dead space, this will ↓ effective alveolar minute ventilation → hypoventilation → potentially cause hypoxaemia
- Apparatus dead space
- If the dead space of a breathing circuit is large relative to the size of the tidal volume, eg in neonates, it can result in significant rebreathing of CO2 and contribute to hypoxaemia - another way to think about it is that if total minute ventilation stays the same, by increasing dead space ventilation, alveolar minute ventilation is reduced, resulting in relative hypoventilation
Measurement
- Fowler's Method - measures anatomical dead space
- Bohr Equation - measures physiological dead space
- See sections below
Fowlers Method
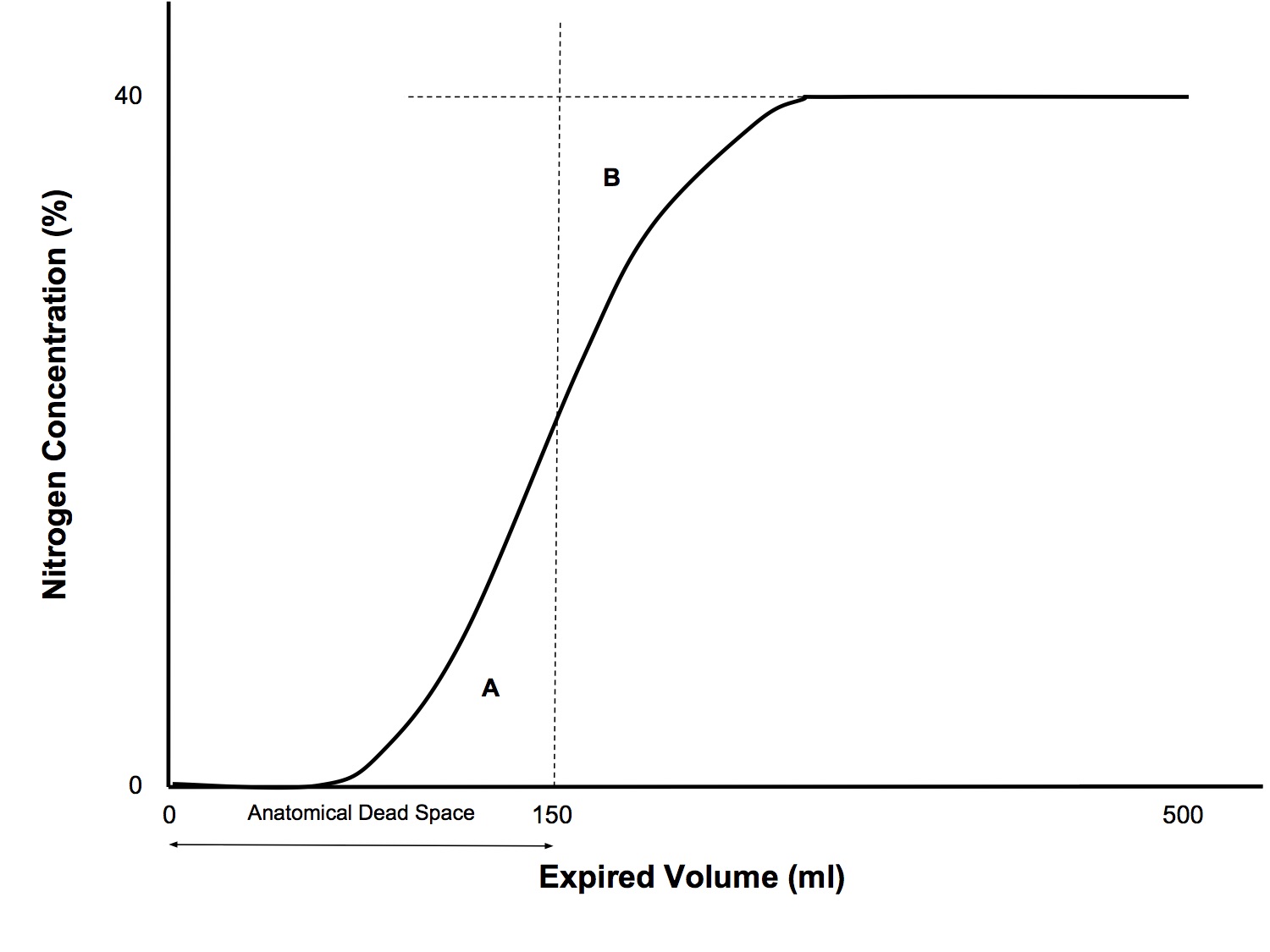
Application
- Measures anatomical dead space
Technique
- Single breath nitrogen washout
- Rapid N2 analyser
- Pneumotachograph to record expired volume
- Single inspiration of 100% O2 followed by expiration
- N2% is plotted against volume
Principles
- At end-inspiration of the 100% O2 breath, the anatomical dead space is filled with 100% O2 and the N2 concentration in the alveoli has been diluted
- The first part of the exhaled gas comes from the anatomical dead space, with zero N2 content
- This is followed by a rapid rise in N2 concentration - the transition between dead space gas and alveolar gas
- This is followed by a plateau, which represent homogenously mixed alveolar gas
- The point where the area of triangle A equals the area of triangle B is taken to represent the exact transition point between anatomical dead space and alveolar gas
Bohr Equation
Vd = PaCO2 - PeCO2
Vt
PaCO2
- Measures physiological dead space
- VD/VT = (PACO2 - PECO2)/PACO2
- Normal value: 0.2~0.35
- NB: PECO2 is the partial pressure in MIXED expired gas, NOT end-tidal gas
- Enghoff modification
- using measured arterial PaCO2 as an estimate of the ideal alveolar PACO2
- VD/VT = (PaCO2 - PECO2)/PaCO2
- See West for an explanation of how this equation is derived
ET - arterial pCO2 Difference
- Gas transfer of CO2 across alveolar membrane is perfusion limited and alveolar and end pulmonary capillary (and hence arterial) pCO2 should be equilibrated
- End tidal CO2 is representative of mixed alveolar gas, however ETCO2 is slightly lower than paCO2 even in healthy people, by ~3mmHg, due to a small amount of alveolar dead space
- Alveolar dead space is West zone 1, where PA > Pa > Pv. Because pulmonary pressures are low, typically 25/8mmHg, because of the effect of gravity on hydrostatic pressures, in the erect position the apex of the lung may or may not be barely perfused
- Alveolar dead space are alveoli that are ventilated but not perfused and hence do not participate in gas exchange, thus they have the same composition as the inspired gas which contains no CO2* (not quite true, see Brandis, but for exam purposes the KISS principle applies!)
- During expiration, alveolar DS gas mixes with the remaining alveolar gas (which contains pCO2 that has equilibrated with arterial pCO2) and slightly dilutes the CO2 concentration
- Any conditions that increase alveolar dead space will increase the ET-arterial CO2 difference, eg pulmonary embolus, high PEEP, low cardiac output states
Shunt
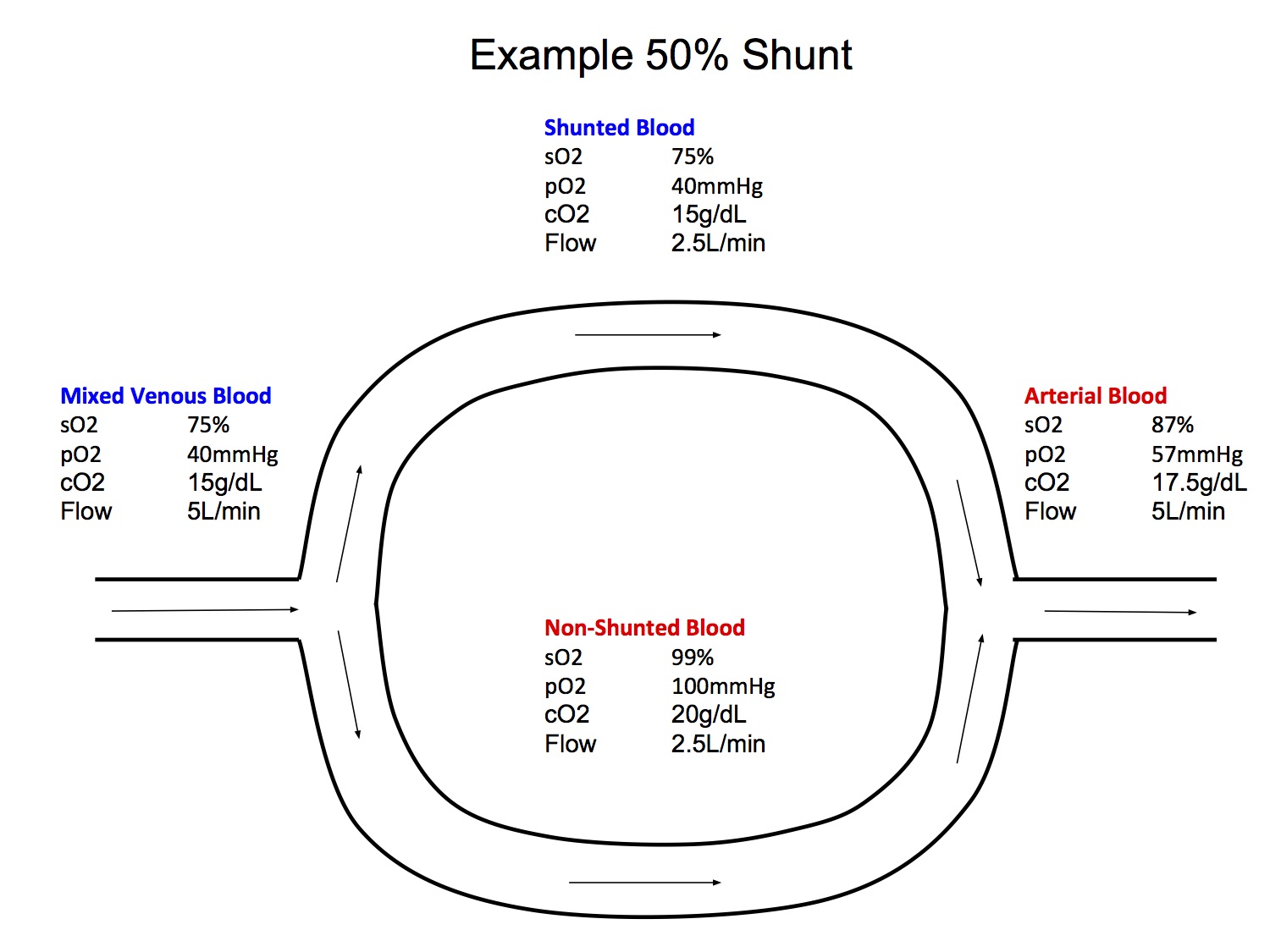
Definition
- Venous blood which enters the arterial system without passing through the gas exchanging parts of the lung
- Physiological
- Bronchial circulation - bronchial arterial blood supplies O2 to bronchi then is drained by the pulmonary veins and return to the left atrium
- Thebesian veins - coronary venous blood which drains directly into the left ventricle
- Pathological
- Areas of lung that are perfused but not ventilated (atelectasis)
Clinical Significance
- Anaesthesia → ↓ FRC below CC → atelectasis → shunting → hypoxaemia
- Once atelectasis has occurred, a recruitment manoeuvre is required to re-expand the collapsed alveoli, followed by addition of PEEP to prevent re-collapse
- When the shunt fraction is > 30%, ↑ FiO2 will no longer improve the hypoxaemia (see isoshunt diagram)
Venous Admixture
- The amount of mixed venous blood that would have to be added to pulmonary end-capillary blood to produce the observed drop in arterial pO2 from the pO2 in end-capillary blood
- Note that not all venous blood from different regions has the same gas composition as mixed venous blood (eg venous blood in thebesian veins has a lower pO2 due to the high O2 extraction ratio of the heart)
- Two sources
- True shunt
- Blood from areas of the lung with low V/Q ratios
Are shunt and venous admixture interchangeable terms?
- No they are not
- They way I think about it is that shunting is a pathophysiological mechanism, while venous admixture is a measurement that quantifies shunt (if you pretend that all shunted blood is equal and that there is no contribution from low V/Q lung units)
- It may seem a bit pedantic, but they are not equivalent and if you mix up these terms in the exam, you will lose marks because it implies that you don't understand that
- venous admixture is not only due to shunt but also low V/Q lung units
- not all shunted blood is the same, because of regional differences in O2 consumption. Coronary O2 extraction is higher than other organs, so shunted blood from the thebesian veins is more deoxygenated than shunt from other sources
Shunt Equation
Qs = Cc'O2 - CaO2
Qt
Cc'O2 - CvO2
Qs
shunt blood flow
Qt
total blood flow
Cc'O2
end pulmonary capillary content of O2
CaO2
arterial content of O2
CvO2
mixed venous content of O2
Despite being called the "shunt equation", it actually calculates venous admixture, not shunt
(see West if you are interested in the derivation of this equation)
Isoshunt Diagram
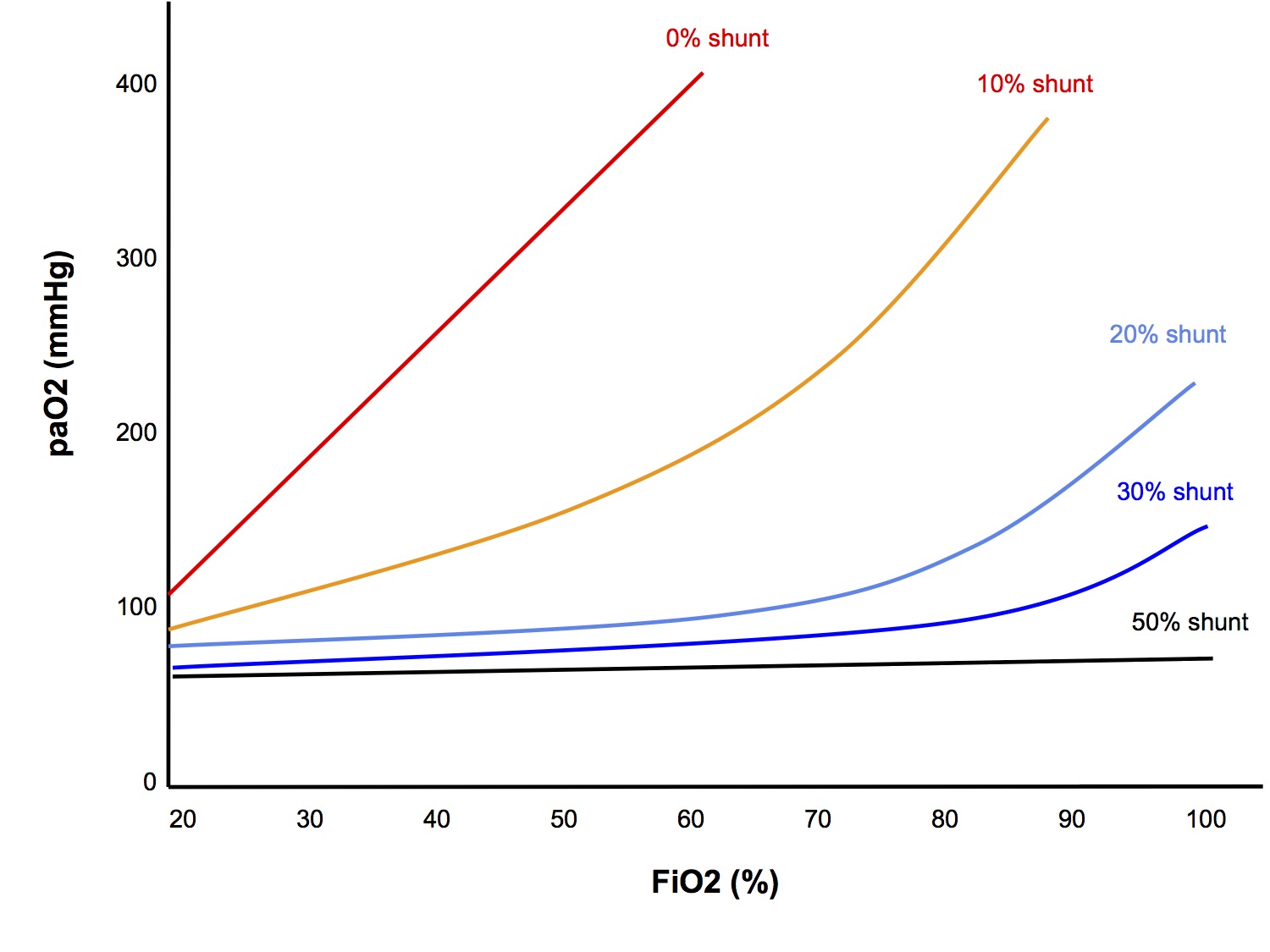
At low levels of shunt, ↑ FiO2 → ↑ paO2, however as the shunt fraction becomes greater than 30%, ↑ FiO2 no longer improves the paO2
See Worked SAQ Answer "Shunt and Hypoxaemia" for detailed explanation
A-a Gradient pO2
- Gas transfer of O2 across alveolar membrane is perfusion limited and alveolar and end pulmonary capillary (and hence arterial) pO2 should be equilibrated
- However, arterial pO2 is slightly lower than pAO2 in a healthy person due to a small amount of shunt and low V/Q lung units, ie venous admixture
- Venous admixture blood is desaturated and dilutes the O2 content in arterial blood causing the A-a gradient
- Conditions that increase true shunt and V/Q mismatch will increase the A-a gradient, such as atelectasis, CAL, old age.
V/Q Mismatch
V/Q mismatch is a concept that many candidates struggle with. It is worth reading the chapter "Ventilation-Perfusion Relationships" in West for a fuller, yet still fairly succinct explanation.
"V/Q mismatch" is often used as an all-encompassing answer to any respiratory physiology question:
How does anaesthesia cause hypoxaemia? - V/Q mismatch
Why does atelectasis cause hypoxaemia? - V/Q mismatch
What causes the A-a gradient? - V/Q mismatch
What causes the ET-a CO2 difference? - V/Q mismatch
While these answers are technically not incorrect, they are very imprecise and uninformative. It's equivalent to saying:
Why did you order this CT scan? - to look for abnormality
What are your management goals for
this critically ill patient requiring major, emergent surgery? - avoid hypoxia and hypotension
Make sure that you have a clear understanding of shunt, alveolar dead space and V/Q mismatch and how they relate to each other.
What is the V/Q ratio?
- The V/Q ratio is the ratio of alveolar ventilation to alveolar perfusion
- This concept can be applied to the lungs as a whole or to individual lung units
- Total minute ventilation = resp rate x tidal volume
15bpm x 500ml = 7500ml/min
- About 1/3 of the tidal volume is dead space, so alveolar minute ventilation =
2/3 x 15 x 500 = 5,000ml/min
- Pulmonary blood flow = cardiac output = 5,000ml/min
- Hence the normal V/Q ratio of the lungs is 1
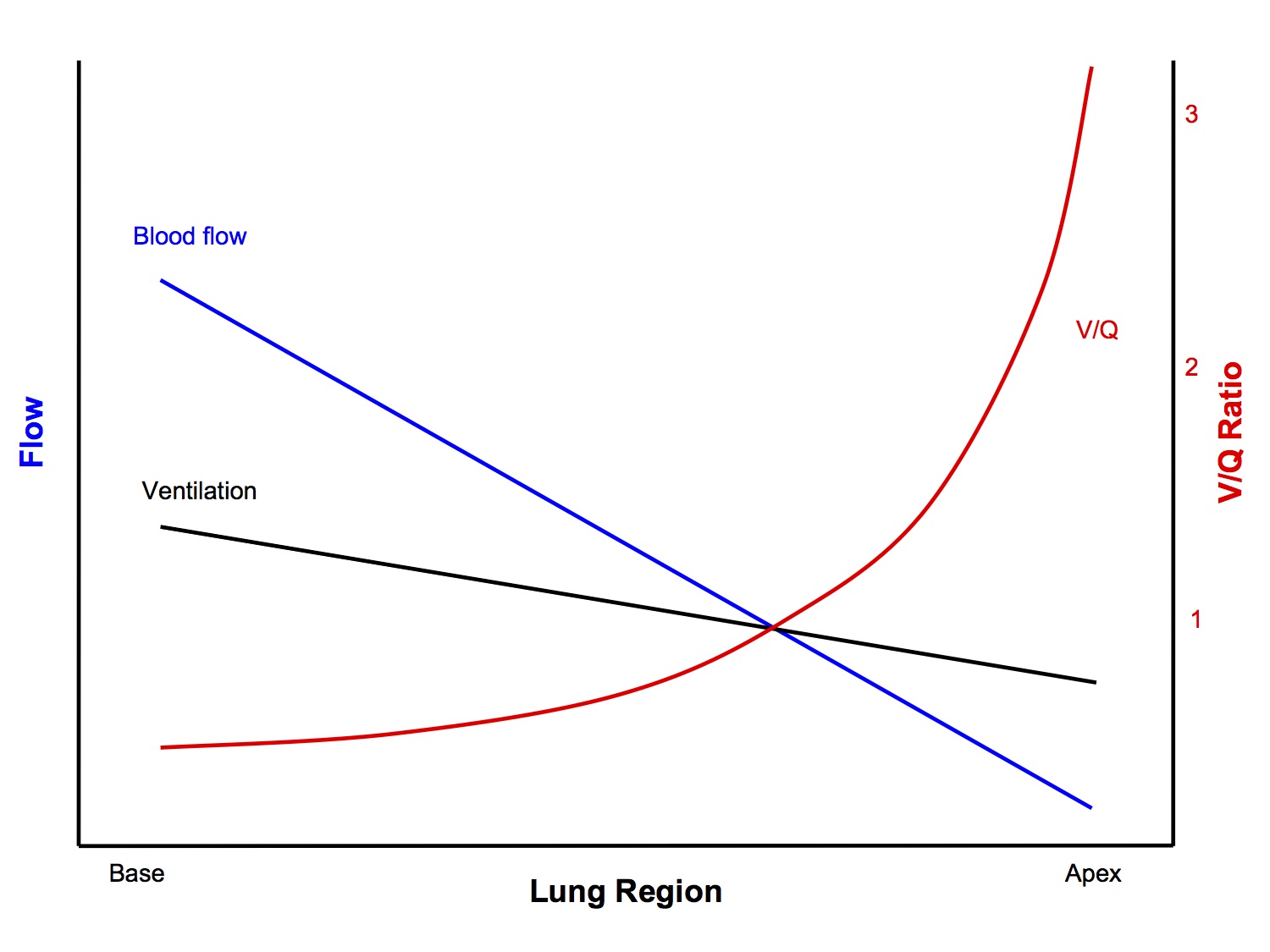
How does V/Q ratio vary from the apex to the base of the lungs?
- The V/Q of an "ideal alveolus" is 1
- However, in reality the V/Q ranges from 3.3 at the apex to 0.63 at the base (in the erect position)
- Why? The important points are:
- Both ventilation and perfusion increase, going from the apex to the base
- However, perfusion increases more than ventilation
- Ventilation
- The weight of the lungs pressing down on itself tends to compress the bases more than the apex
- This means that the basal alveoli operate at a smaller volume than the apical alveoli, which are more stretched open
- This results in the basal alveoli being on the steeper, middle part of the compliance curve while the apical alveoli are on the flatter, upper part
- Hence, during inspiration, the base receives more ventilation than the apex
- Note that if lung volumes are reduced (the classic "what happens if FRC is reduced by 1L" SAQ), then the apical alveoli are shifted down to the steeper, middle part of the compliance curve and the basal alveoli are shifted down to the flatter, lower part, resulting in a reversal of the normal ventilation distribution pattern
- Perfusion
- Due to the effect of gravity on hydrostatic pressures, and the fact that the pulmonary circulation is a low pressure system, perfusion is greater in the bases
- See section on West Zones for a more detailed explanation
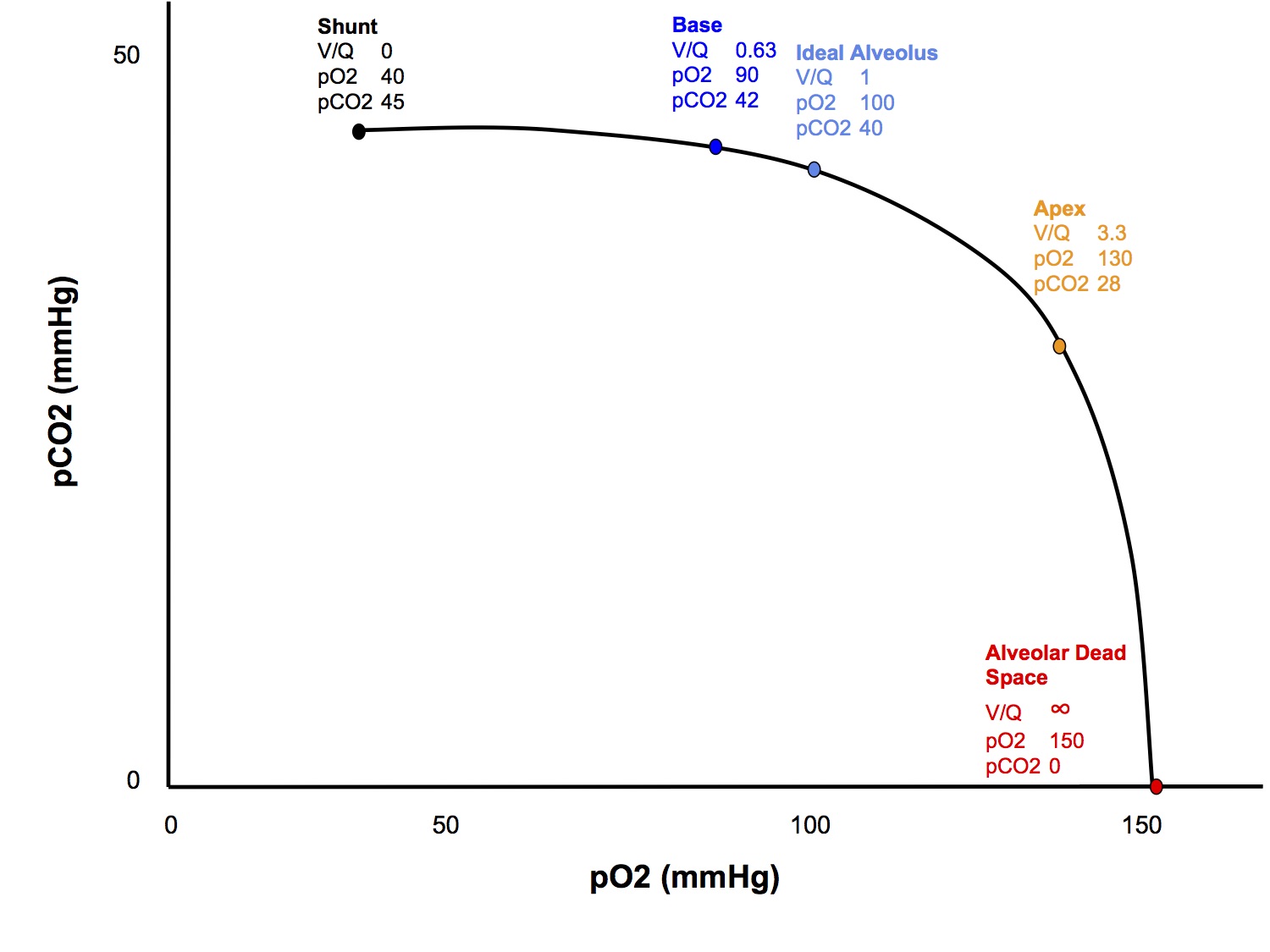
What is V/Q mismatch?
- Any deviation in the ventilation to perfusion ratio away from the ideal V/Q of 1
- Encompasses a spectrum from V/Q 0 (shunt) to V/Q ∞ (alveolar dead space)
- Shunted blood, by definition, passes through alveoli that are not ventilated, hence cannot participate in gas exchange, hence has the same gas composition as venous blood (pO2 40, pCO2 45) and passes into the arterial circulation
- Inspired gas that passes down into alveolar dead space, by definition, cannot participate in gas exchange because there is no perfusion to that area, so it retains the same composition as the inspired gas (pO2 150 if room air, pCO2 0), then is exhaled during expiration
- Between the extremes of alveolar dead space and shunt, the gas composition will vary according to the V/Q ratio
- The gas composition of blood passing through low V/Q areas approaches that of shunt ( like mixed venous blood) , while blood passing through high V/Q areas will approach that of alveolar dead space (like the inspired gas)
|
| Dead space | Apex | Ideal | Base | Shunt |
| V/Q ratio | ∞ | 3.3 | 1 | 0.63 | 0 |
| pO2 | 150 | 130 | 100 | 90 | 40 |
| pCO2 | 0 | 28 | 40 | 42 | 45 |
|
| Composition same as room air |
|
|
| Composition same as mixed venous blood |
If the normal lung contains units with V/Qs that range from 0.63 to 3.3, with different gas compositions, how can we say that normal alveolar pO2 is 100mmHg and normal alveolar pCO2 is 40mmHg? And why, then, on a capnograph trace, do we get a homogenous plateau of CO2 concentration representing alveolar gas?
- For simplicity, we tend to view the lungs as if they were one big ideal alveolus, while in reality they are quite heterogeneous
- Fortunately, in practice, in a healthy lung, during expiration the alveolar gas from different V/Q areas mix quite thoroughly in the conducting airways and results in a fairly uniform "average" gas composition, so our oversimplified view has some utility
- During disease states, when different lung units can empty at different rates (fast/slow alveoli), this can unmask the heterogeneity, eg the upsloping capnograph seen with obstructive lung disease
Hypoxic Pulmonary Vasoconstriction
- V/Q mismatch is normally minimised by the process of HPV
- The smooth muscle in the walls of small pulmonary arterioles constricts in the presence of low alveolar pO2
- This tends to reduce blood flow to lung regions with poor ventilation and improve V/Q matching
- The exact mechanism is not known
- May involve nitric oxide (v/d) and endothelins (v/c)
- It is a local reflex that does not require neural control
- It occurs in response to low alveolar pO2, not low pulmonary arterial pO2
- Volatile anaesthetic agents completely inhibit HPV at > 1 MAC, however the clinical significance of this is disputed
What effect does increased V/Q mismatch have on paO2?
- See section in Worked SAQ Answers, V/Q Mismatch and Hypoxia
- In a nutshell, increased V/Q mismatch causes hypoxaemia because
- More blood passes through areas of low V/Q than areas of high V/Q
- Due to the shape of the O2 Hb dissociation curve, blood from high V/Q areas (with marginally increased O2 content) cannot compensate for blood from low V/Q areas (with significantly decreased O2 content)
What effect does increased V/Q mismatch have on paCO2?
- Potentially can increase paCO2, but much less effect than on paO2
- This is because
- The shape of the CO2 Hb dissociation curve is much more linear, so that high V/Q areas can compensate for low V/Q areas
- Any increase in paCO2 is detected by the chemoreceptors and stimulates increased ventilation
O2 - CO2 V/Q Diagram

West Zones
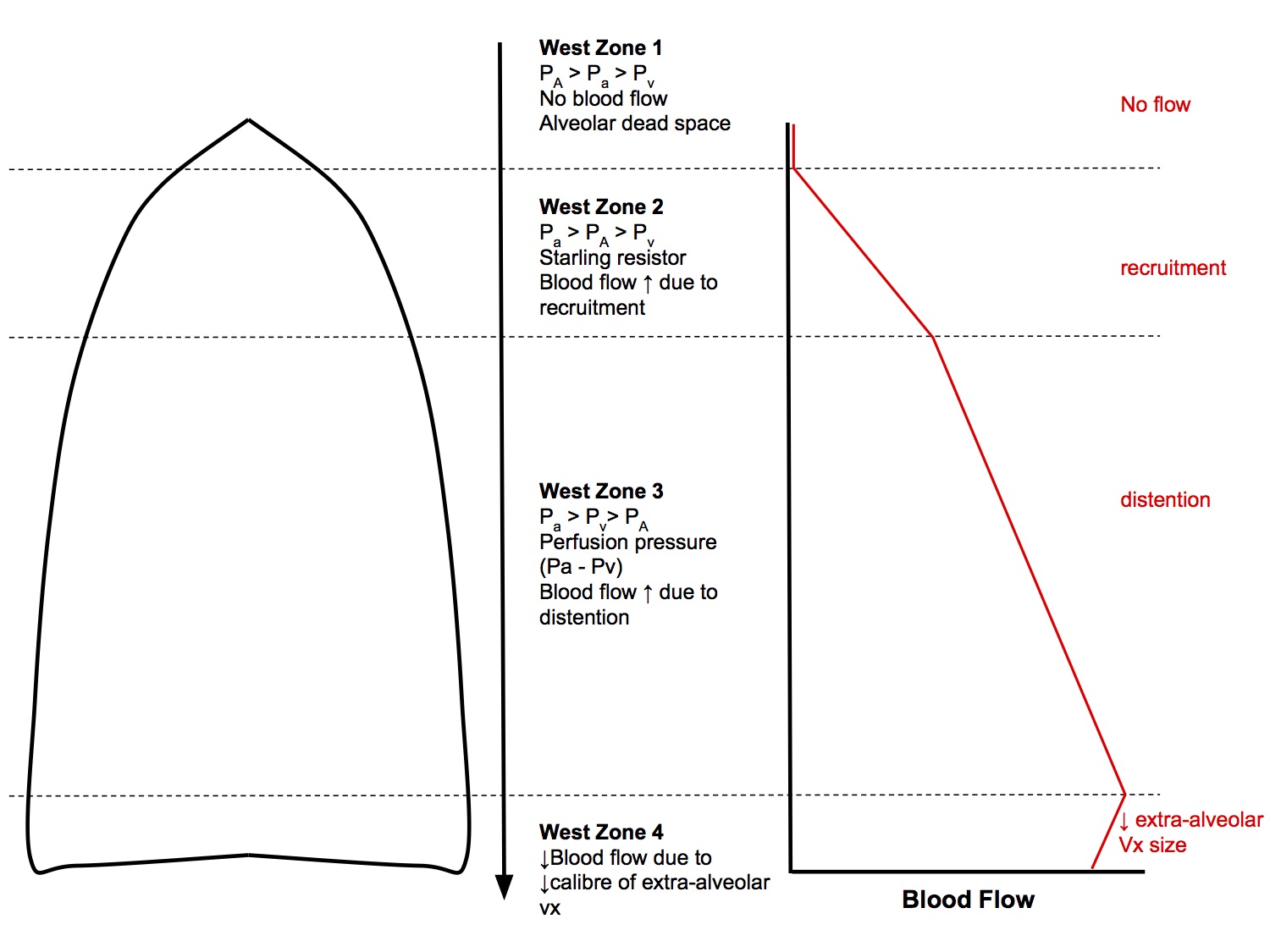
Going from the apex to the base of the lung (in the erect position), pulmonary arterial pressure increases due to the effects of gravity on hydrostatic pressure - moving up or down 30cm equates to a change in arterial pressure of 23mmHg. This results in an interesting distribution of blood flow. Areas of differing patterns of blood flow are classified into West Zones:
West Zone 1
PA > Pa > Pv
- Occurs at the apex of the lung (or the non-dependant part if in a non-erect position)
- Pulmonary pressures are low, typically quoted as 25/8mmHg at the heart level
- If at the lung apex, arterial pressure drops below alveolar pressure, then the capillaries are squashed flat and it becomes a region that is ventilated but not perfused, ie alveolar dead space
- West states that West Zone 1 does not occur in the healthy state, however Brandis suggests that it does and is the explanation for the small ET-arterial CO2 difference that can occur even in healthy people. For exam purposes, it is easier to side with Brandis on this!
West Zone 2
Pa > PA > Pv
- Perfusion pressure in this zone is arterial minus the surrounding alveolar pressure, rather than the downstream venous pressure
- This is a Starling resistor, analogous to cerebral perfusion pressure when ICP is increased or dynamic airway compression
- Blood flow will vary during the respiratory cycle as alveolar pressures change
- Going down the lung, arterial pressures increase, due to effects of gravity on hydrostatic pressure, while alveolar pressure remains the same, leading to increasing blood flow towards the base of the lungs. Recruitment of intra-alveolar capillaries also contributes to ↑ blood flow
West Zone 3
Pa > Pv > PA
- The majority of the lung, where perfusion pressure is equal to arterial minus venous pressure
- Going down the lung, blood flow increases mainly due to distention of intra-alveolar capillaries
West Zone 4
- Region of reduced blood flow at the base of the lungs. This is due to the weight of the lung compressing the base → smaller lung volume → ↓ radial traction holding extra - alveolar vessels open
- This is analogous to atelectasis occurring at the base because of ↓ radial traction holding the small airways open
Hypoxic Pulmonary Vasoconstriction
- HPV is a homeostatic mechanism that aims to maintain optimal V/Q matching by diverting blood away from poorly ventilated areas of lung
- Small pulmonary arteries constrict in response to low alveolar pO2 (not low blood pO2)
- Mechanism involves nitric oxide (potent vasodilator) that is continuously synthesised by the pulmonary vascular endothelium. Synthesis of NO reduces when pAO2 drops below 70mmHg
- Clinical significance
- Helps reduce V/Q mismatch and prevent hypoxaemia
- At birth, after the first breath reduction in HPV results in increased pulmonary blood flow in the neonate
- Volatile anaesthetic agents above 1 MAC inhibit HPV (though the clinical significance of this is disputed)
Regional Differences
Apex vs Base
If you are asked a question relating to the difference between the apex and base of the lungs, in the erect position - which is pretty much the same as asking for the differences between the non-dependent and dependent parts of the lung in any position - there are several things you should comment on, depending on the scope of the question:
- Ventilation
- Perfusion
- V/Q ratio
- Gas composition
- Compliance
- Fast and slow alveoli
Ventilation, Perfusion and V/Q Ratio
Ventilation
- The weight of the lungs pressing down on itself tends to compress the bases more than the apex
- This means that the basal alveoli operate at a smaller volume than the apical alveoli, which are more stretched open
- This results in the basal alveoli being on the steeper, middle part of the compliance curve while the apical alveoli are on the flatter, upper part
- Hence, during inspiration, the base receives more ventilation than the apex
- Note that if lung volumes are reduced (the classic "what happens if FRC is reduced by 1L" SAQ), then the apical alveoli are shifted down to the steeper, middle part of the compliance curve and the basal alveoli are shifted down to the flatter, lower part, resulting in a reversal of the normal ventilation distribution pattern
Perfusion
- Due to the effect of gravity on hydrostatic pressures, and the fact that the pulmonary circulation is a low pressure system, perfusion is greater in the bases
- See section on West Zones for a more detailed explanation
V/Q Ratio
- V/Q ratio ranges from 3.3 at the apex to 0.63 at the base (in the erect position)
- Why? The important points are:
- Both ventilation and perfusion increase, going from the apex to the base
- However, perfusion increases more than ventilation

Gas Composition

|
| Dead space | Apex | Ideal | Base | Shunt |
| V/Q ratio | ∞ | 3.3 | 1 | 0.63 | 0 |
| pO2 | 150 | 130 | 100 | 90 | 40 |
| pCO2 | 0 | 28 | 40 | 42 | 45 |
|
| Composition same as room air |
|
|
| Composition same as mixed venous blood |
Compliance
- The weight of the lungs pressing down on itself tends to compress the bases more than the apex
- This means that the basal alveoli operate at a smaller volume than the apical alveoli, which are more stretched open
- This results in the basal alveoli being on the steeper, middle part of the compliance curve while the apical alveoli are on the flatter, upper part
- Hence, during inspiration, the base receives more ventilation than the apex
- Note that if lung volumes are reduced (the classic "what happens if FRC is reduced by 1L" SAQ), then the apical alveoli are shifted down to the steeper, middle part of the compliance curve and the basal alveoli are shifted down to the flatter, lower part, resulting in a reversal of the normal ventilation distribution pattern
Fast and Slow Alveoli
Time Constants
- The time constant is a concept that describes the time course of an exponential process
- One time constant is the time taken for an exponential process to be 63% complete
- After 3 time constants, an exponential process will be 95% complete
Alveolar Filling & Emptying
- Filling and emptying of alveoli are exponential processes (wash in and wash out functions)
- The time constant for an alveolus is mathematically equal to resistance x compliance
- resistance = resistance to airflow of the bronchiole feeding into the alveolus
- compliance = compliance of the alveolus
- The time constant for a "normal" alveolus is 0.2secs
Fast vs Slow Alveoli
- Fast alveoli
- LOW resistance and/or LOW compliance
- Fast to fill/empty completely
- Slow alveoli
- HIGH resistance and/or HIGH compliance
- Slow to fill/empty completely
Normal Lung - Regional Variations
- Lung units in the apex have a lower compliance and lower airway resistance than lung units at the base, in the erect position (see section Regional Differences → Apex vs Base)
- Hence, faster alveoli in apex and slower alveoli in base
Pathological Dispersion of Time Constants
- Analogous to how lung disease can cause increased V/Q mismatch, it can also cause increased variation in the time constants of alveoli
- Emphysema → destruction of supportive connective tissue → ↑ compliance → longer time constants → more slow alveoli
- Asthma → bronchoconstriction → ↑ airway resistance → longer time constants → more slow alveoli
- Pulmonary fibrosis → ↓ compliance → more fast alveoli
Clinical manifestations
- Upsloping "plateau" phase of capnograph
- Fast alveoli empty earlier in expiration while slow alveoli empty towards the end of expiration
- Gas from fast alveoli has lower pO2 while gas from slow alveoli has higher pCO2 (read Nunn's if you want an explanation of the mechanism for this)
- In normal lungs, the variation between fast and slow alveoli is small and the pCO2 during expiration remains fairly constant or only slightly rises
- In disease states, the variation is exaggerated, resulting in higher pCO2 towards the end of expiration and upsloping of the capnograph trace
- ↓ Dynamic lung compliance
- Dynamic compliance is measured without any breath holds to allow for equilibration (remember it takes > 3 time constants for an alveolus to completely fill/empty, which may be a long time for a diseased slow alveolus)
- By the end of inspiration, slow alveoli may not have finished filling yet
- At the start of expiration, gas from fast alveoli may empty into neighbouring slow alveoli, rather than exiting the lungs (this redistribution of gas is called pendelluft, see West or Nunn's)
- This results in a reduced measured expired volume, thus decreasing the calculated compliance
- ↑ Difference between peak and plateau pressures
- Because pendelluft affects dynamic but not static compliance, this results in a greater differential between the peak and plateau airway pressures
- At faster respiratory rates, this allows less time for slow alveoli to equilibrate and accentuates the difference between peak and plateau pressures
- See sections Mechanics of Breathing → Time Dependent Pulmonary Elastic Behaviour and Measurement of Compliance
Gas Transport
Oxygen Transport
O2 is transported in two forms:
- Bound to Hb (major)
- Dissolved (minor)
Haemoglobin
- Haem is iron-porphyrin compound, and is joined to protein globulin, which consists of 4 polypeptide chains (2 alpha, 2 beta)
- Each Hb consists of 4 haem groups and 4 globulin chains and can carry 4 O2 molecules
- Hufner's no (k): amount of O2 that can combine with 1g of Hb
- k = 1.34ml O2/g
- If Hb conc 15g/dL, then haemoglobin will carry 15 x 1.34 = 20ml O2 / dL blood
- Subtypes
- HbA adult
- HbF fetal
- HbS sickle cell
Dissolved O2
- Obeys Henry's Law (amount dissolved is proportional to partial pressure
- 0.003ml O2 / mmHg / dL blood
- If pO2 100mmHg, dissolved O2 contributes 0.3ml O2 / dL blood
Oxygen Flux Equation
| O2 flux | = Chemical O2 delivery + Dissolved O2 delivery |
| = CO x [Hb] x SaO2 x k + CO x paO2 x 0.003 | |
| = 50dL/min x 15g/dL x 0.99 x 1.34ml O2/g + 50dL/min x 100mmHg x 0.003ml O2/mmHg/dL | |
| = 1000ml O2 / min |
Put the Numbers in Perspective
- O2 delivery via the cardiac output is 1000ml/min
- O2 consumption from BMR is 250ml/min, ie 1/4 of delivered O2 is consumed
- This is why arterial sO2 is 100% and venous sO2 is 75%
- Breathing room air, Hb contributes 20ml O2/dL while dissolved O2 contributes 0.3ml O2/dL
- Hence O2 carriage in blood under normal circumstances is
- 98.5% bound to Hb
- 1.5% dissolved
- If a patient is anaemic, with Hb 10g/dL and hypoxaemic, with saO2 80% and breathing 100% O2
- Hb will contribute 10 x 0.8 x 1.34 = 10.7ml O2/dL
- Dissolved O2 will contribute 0.003 x 760 = 2.3ml O2/dL
- 82.3% bound to Hb
- 17.7% dissolved
- Hb is still more important, but dissolved O2 starts to become more significant
O2-Hb Dissociation Curve
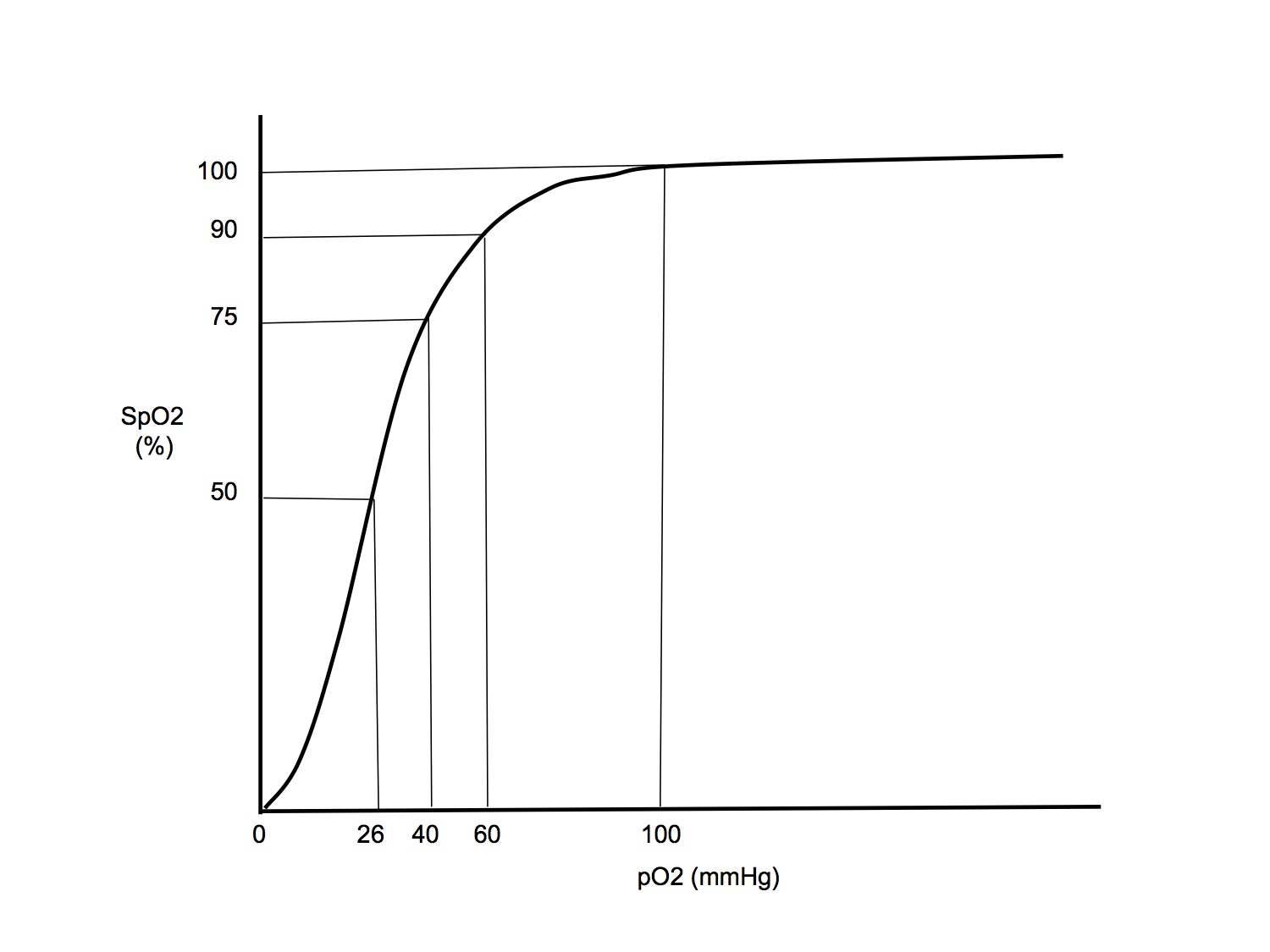
- ODC is sigmoid shaped
- Important points on the curve
| sO2(%) | pO2(mmHg) | Comment |
| 98 | 100 | Normal arterial value |
| 90 | 60 | The "ICU point". Below pO2 60, a small drop in pO2 → large drop in sO2 and O2 content. Hence in ICU, always aim for target pO2 > 60mmHg |
| 75 | 40 | The mixed venous point. Common question in the vivas is to ask if this point is actually on the same ODC as the arterial point - it is not, as venous blood has higher pCO2 hence the curve would be right shifted |
| 50 | 26.6 | The P50, where sO2 = 50%. Compare this with foetal HbF with P50 that is shifted to the left with pO2 18mmHg |
| 10 | 10 | Just easy to remember |
- Right shifted when ↑ in: (helps offload O2 in tissues)
- Temperature
- [H+]
- pCO2 (Haldane effect)
- Red cell 2,3 DPG (end product of rbc metabolism. Increased in chronic hypoxia)
- Positive cooperativity:
- When a globin chain is oxygenated, it causes structural changes which increase the affinity of the haem of the remaining chains for O2
- This increases O2 affinity as O2 loads and causes the sigmoid shape
- Significance of shape:
- Flat upper portion means if pAO2 drops a bit, O2 loading is unaffected. Also, it maintains a large partial pressure difference when most of O2 has been transferred, hastening diffusion
- Steep lower part means tissue can withdraw a large amount of O2 for only small drop in pO2, assisting diffusion
Functional vs Fractional SaO2
- Functional SaO2 = [HbO] / ([HbO] + [Hbdeoxy])
- Fractional SaO2 = [HbO] / ([HbO] + [Hbdeoxy] + [MetHb] + [COHb])
CO2 Transport
- CO2 is carried in blood in 3 forms:
- Dissolved
- Bicarbonate
- Carbamino compounds
- Arterial blood contains 48ml CO2 / dL
- Mixed venous blood contains 52ml CO2 / dL
|
| % total carriage | % contribution to a-v diff |
| Dissolved | 5 | 10 |
| Bicarbonate | 90 | 60 |
| Carbamino compounds | 5 | 30 |
Dissolved
- Obeys Henry's Law
- The amount of gas dissolved in a liquid is proportional to the partial pressure of the gas that is in equilibrium with the liquid, at a constant temperature
- CO2 is 20 x more soluble than O2
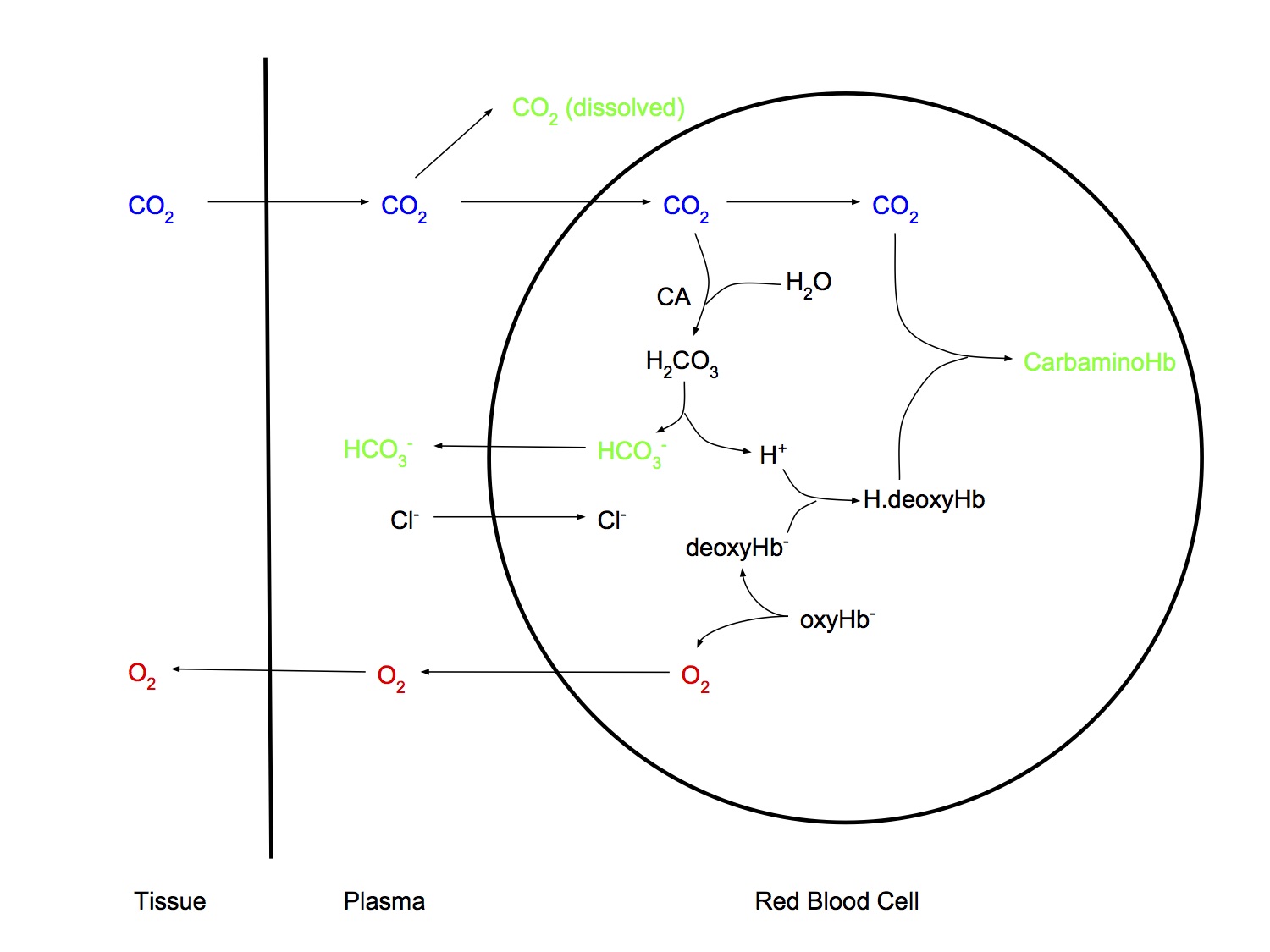
Bicarbonate
- CO2 + H2O < => H2CO3 < => H+ + HCO3-
- First reaction slow in plasma but fast in rbc due to carbonic anhydrase
- Chloride shift as HCO3- exits rbc and Cl- enters
Carbamino compounds
- Formed from CO2 reacting with
- Terminal amino groups of proteins
- Amino groups in side chains of argininge and lysine
- Hb is more important than plasma proteins for carriage of CO2 in carbamino form because
- More Hb present (15g/dL vs 7g/dL)
- Hb is tetramer with 4 N-terminal groups, while albumin has only 1
- Deoxygenation of Hb in the tissues augments the formation of carbamino-Hb (Haldane effect)
Haldane Effect
- Describes the phenomenon whereby deoxygenation of haemoglobin increases the ability of blood to carry CO2
- O2 unloading assists with CO2 loading in blood
- This is due to the fact that deoxyHb is
- 3.5 times more effective than oxyHb at forming carbamino compounds (70% of Haldane Effect)
- A better buffer than oxyHb. Can mop up H+ from dissociation of carbonic acid (30% of Haldane Effect)
CO2 Dissociation curve vs ODC
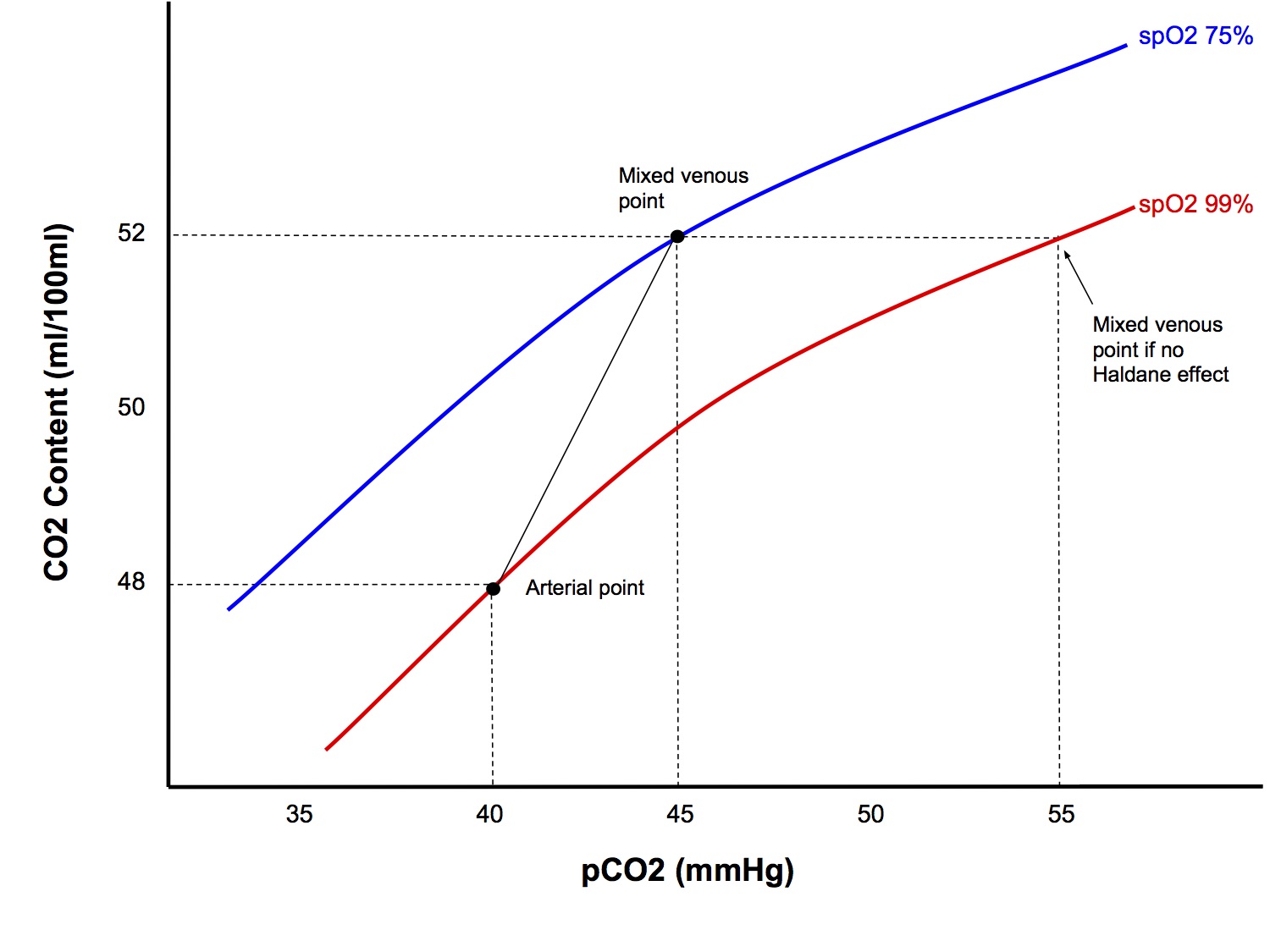
CO2 Content Curve

- Compared to the O2 dissociation curve, it is
- More linear
- Much steeper than for O2
- The Haldane effect shifts the curve upwards
- The above factors explain why a-v difference for pCO2 is much smaller than for pO2
Hypoxaemia & Hypoxia
Hypoxaemia
Definition
- Abnormally low pO2 in arterial blood (pO2 < 60mmHg)
Pathophysiological Mechanisms Causing Hypoxaemia
- Low inspired O2 concentration
- Hypoventilation
- Diffusion problem
- Shunt
- V/Q mismatch
- Diffusion hypoxia (N2O)
Explaining the Mechanisms of Hypoxaemia
- Low FiO2 and hypoventilation
- Using the alveolar gas equation is a good way to illustrate how these mechanisms cause hypoxaemia
- Remember that paCO2 and alveolar minute ventilation are inversely related, so that if minute ventilation halves, then paCO2 will double
- Diffusion problem
- A diffusion problem across the alveolar membrane - such as from consolidation or pulmonary oedema - may result in O2 transfer become diffusion limited instead of perfusion limited
- Shunt
- Explain how deoxygenated venous blood (sO2 75%) bypasses the gas exchanging parts of the lung and dilutes the oxygen content in arterial blood
- Quote the shunt equation
- V/Q mismatch
- Explain how high V/Q units cannot compensate for low V/Q units
- See section Ventilation-Perfusion Relationships → V/Q Mismatch
- Diffusion hypoxia
- Explain how this is a reverse of the second gas effect
Hypoxia
Definition
- Oxygen delivery to the tissues that is inadequate to meet its metabolic demands
Classification
- Hypoxic hypoxia
- Hypoxia due to hypoxaemia
- Who came up with such a silly, confusing name??
- Anaemic hypoxia
- Anaemia causing reduced O2 carrying capacity
- Stagnant hypoxia
- Shock with failure to deliver oxygen to the tissues
- Histotoxic hypoxia
- Inability of the tissues to utilise O2, even if it is present at adequate concentrations
- Cyanide toxicity
Hypoxaemia vs Hypoxia
- As you can see, the two terms are related but not interchangeable, similar to acidosis and acidaemia
- Hypoxaemia is only a subset of the causes of hypoxia
- Use the two term correctly or it sounds sloppy
Forced Expiratory Flow-Volume Loops
Normal Pattern
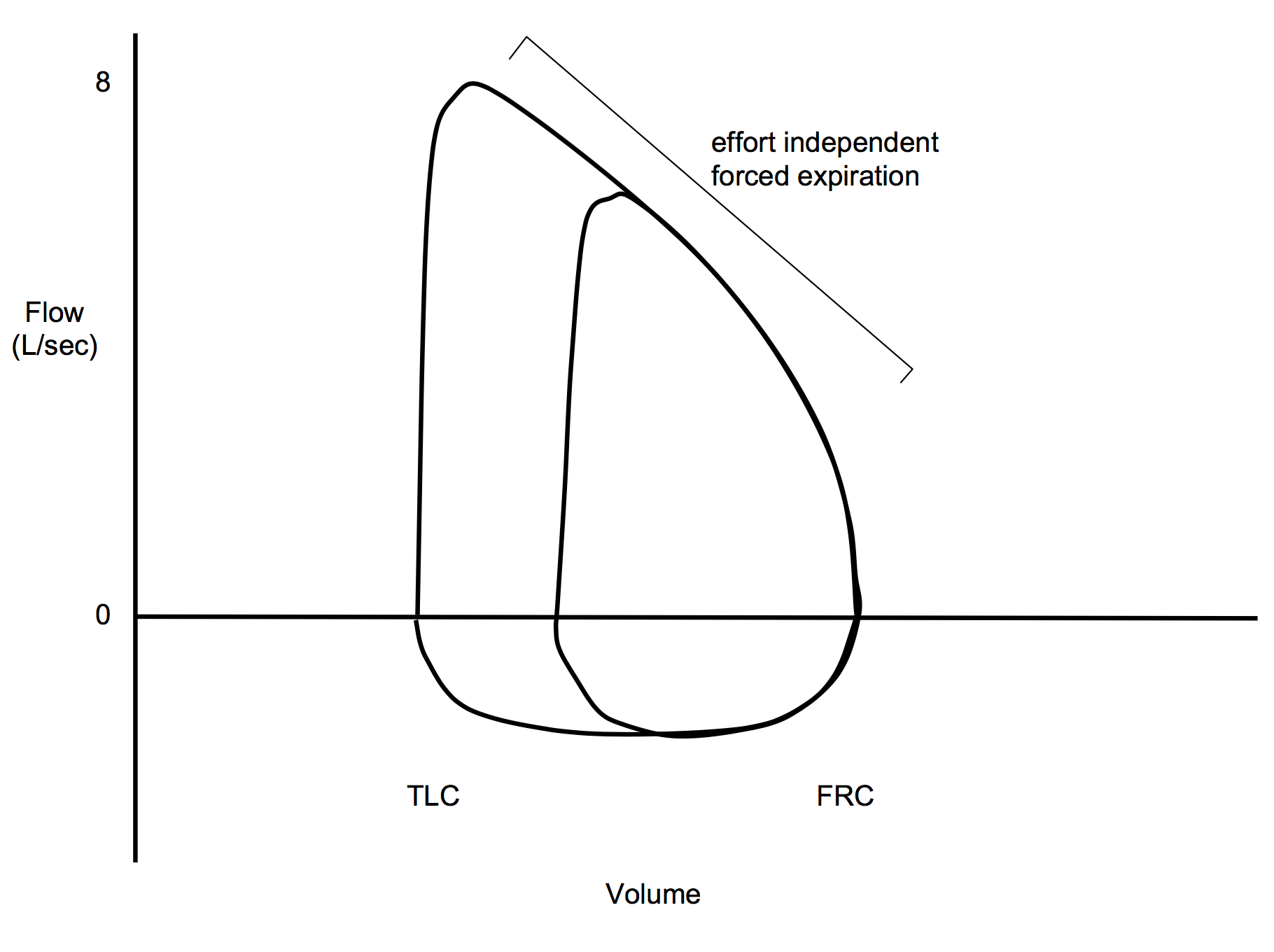
- During forced expiration, after the initial effort dependent rise in flow rate to maximum, the flow rate becomes effort independent, ie ↑ effort does not result in ↑ flow rate
- This is due to dynamic compression of the airways
- During passive expiration, the driving pressure for gas flow is (alveolar P – mouth P)
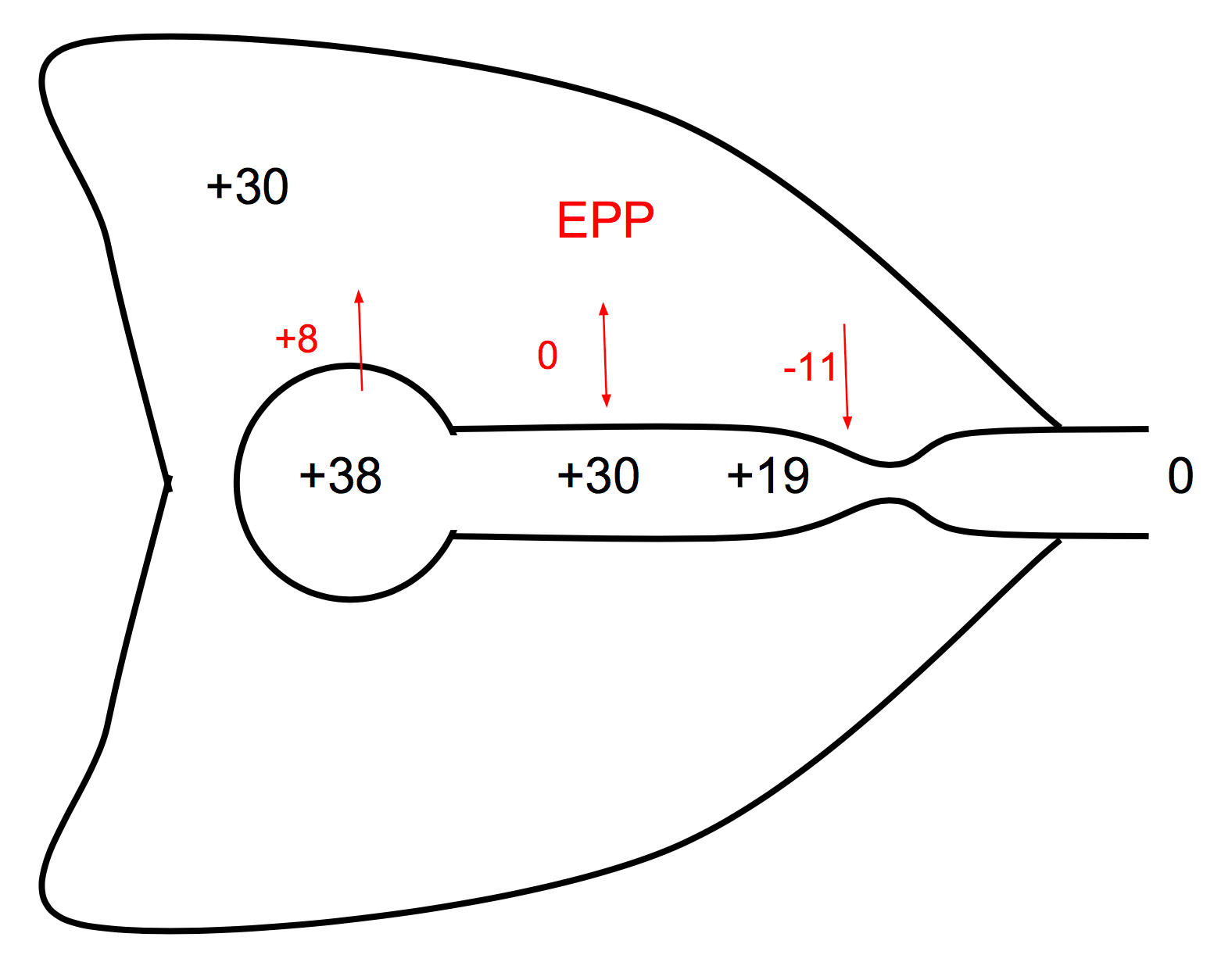
- During forced expiration, the high intrathoracic (or intrapleural) pressure that surrounds the airway will at some point equal then exceed the pressure within the airway lumen as pressure drops from alveolus towards the mouth due to conversion to kinetic energy for gas flow – this is the equal pressure point (EPP)
- Distal to the EPP the airway is narrowed by the higher extraluminal pressure
- Thus the driving pressure becomes (alveolar P – intrathoracic P), ie a Starling resistor
- Increasing expiratory effort increases alveolar and intrathoracic pressures equally, thus the effective driving pressure remains unchanged
- Thus, flow rate depends only on the elastic recoil property of the lung and the lung volume
Abnormal Patterns
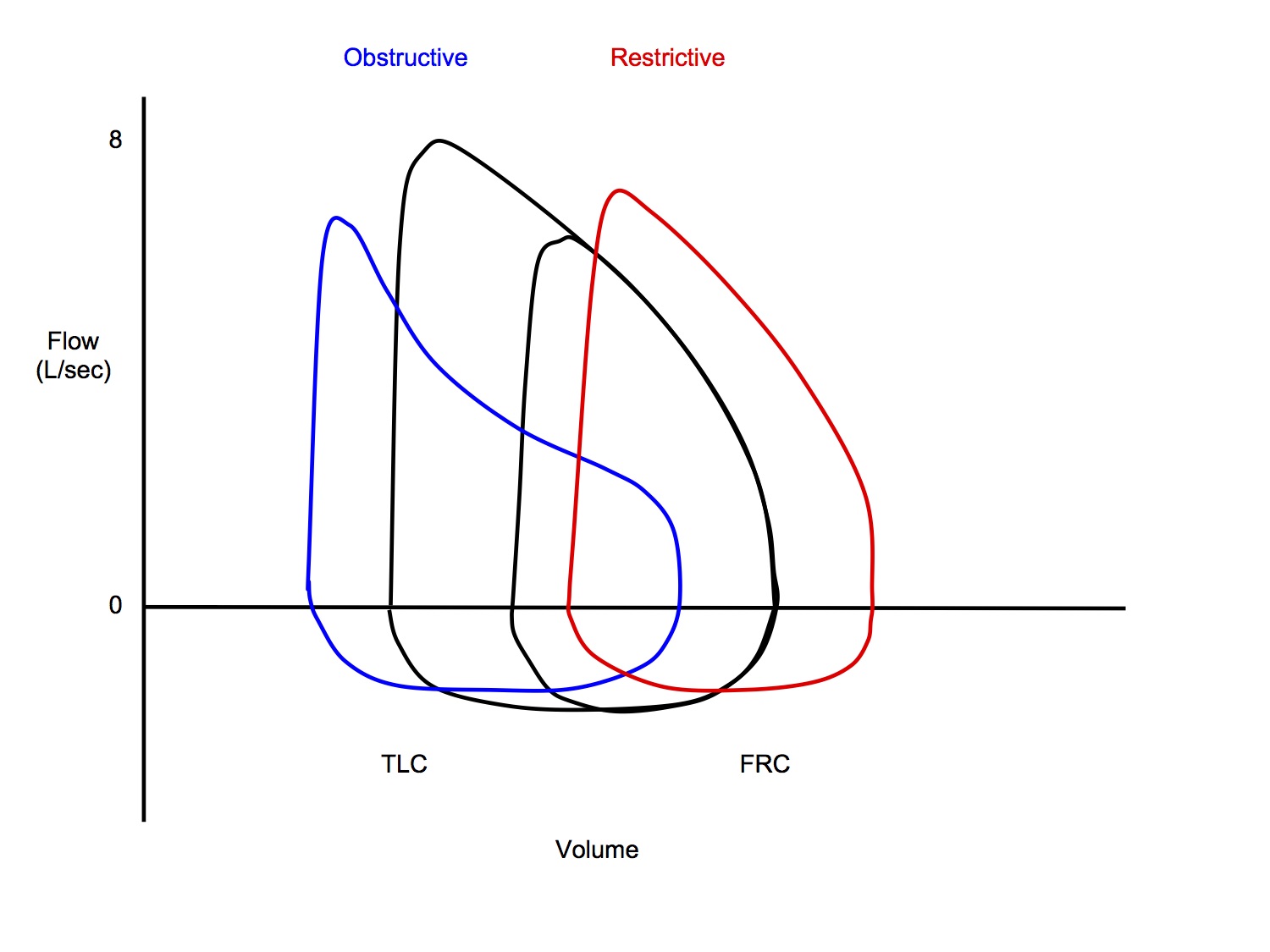
Obstructive (emphysema/chronic bronchitis)
- Starts at larger lung volume
- Destruction of elastic alveolar walls → ↓ static recoil of lungs
- Lower flow rates at the same lung volume
- ↑ airway resistance to flow due to
- airway wall thickening and excessive secretions
- ↓ no of small airways due to tissue destruction
- loss of lung parenchyma → ↓ radial traction on airways → collapse of airways
Restrictive (interstitial fibrosis)
- Starts at smaller lung volume
- Reduced compliance of lungs
- Higher flow rates at the same lung volume
- Static recoil of lungs in higher, hence greater flow rates during the effort independent part or expiration
Worked SAQ Answers
Shunt and Hypoxaemia
Why does increasing FiO2 not improve hypoxaemia if there is a large shunt?
Part A Setup – describing what shunt is and how it causes hypoxaemia
Definition
- Shunt occurs when deoxygenated venous blood enters the systemic arterial circulation without passing through gas exchanging parts of the lung
Sequential explanation of pathophysiological mechanism
- This shunted blood has low O2 saturation (sO2 75% in mixed venous blood) and when it mixes with the oxygenated, non-shunted blood, it dilutes the overall O2 content, thus causing hypoxaemia

Diagram makes explanation faster and clearer and also illustrates understanding of the mechanism (see shunt example diagram)
Part B Answering the actual question asked - explaining why increased FiO2 doesn’ t help that much
- ↑ FiO2 → ↑ pO2 in non-shunted fraction (up to max ~663mmHg with FiO2 100%)
- according to the alveolar gas equation
pAO2 = FiO2 . (PB – PH2O) – pCO2/R
- However, this would not increase the O2 content of the blood by much as above pO2 100mmHg is on flat part of O2 dissociation curve
- This is because most of the O2 content in blood is bound to Hb, with only a small contribution from dissolved O2, in accordance with the O2 flux equation:
CO ([Hb] x SaO2 x 1.34 + paO2 x 0.003)
- Thus when shunted and non-shunted fractions mix, the overall O2 content (and saO2) will only be marginally improved
- When FiO2 ↑ to maximum 100%, dissolved O2 makes more significant contribution to O2 content (1.9ml/dL), but this is still minor
V/Q Mismatch and Hypoxaemia
Why does V/Q Mismatch cause hypoxaemia?
Setup – this part is probably actually the bigger part of the answer, and is necessary to demonstrate understanding of the pathophysiological mechanisms
- An ideal lung unit has a V/Q ratio of 1, ie alveolar ventilation matches perfusion (both ~5000ml/min)
- However, a real lung contains units with V/Qs ranging from high V/Q 3.3 at apex to low V/Q 0.63 at base
- Pathological conditions may cause ↑ V/Q mismatch, ie more lung units with high V/Q, up to ∞ (alveolar dead space) and low V/Q, down to 0 (shunt)
- Lung units with different V/Q have differing gas compositions:
| Dead space | Apex | Ideal | Base | Shunt | |
|---|---|---|---|---|---|
| V/Q ratio | ∞ | 3.3 | 1 | 0.63 | 0 |
| pO2 | 150 | 130 | 100 | 90 | 40 |
| pCO2 | 0 | 28 | 40 | 42 | 45 |
| Composition same as room air | Composition same as mixed venous blood |

Answering the actual question – These 2 lines are the real answer to the question, but if you just wrote this without the above intro, it would look rote learnt, you haven’ t demonstrated your understanding
- V/Q mismatch causes hypoxaemia for 2 reasons:
- Due to the effects of gravity on hydrostatic pressure, more of the lung blood flow comes from the base of the lung where V/Q is lower and pO2 and O2 content is lower
- Lung units with high V/Q contain higher pO2, however, their O2 content is only marginally higher due to the shape of the O2-Hb dissociation curve

This is because most of the O2 content in blood is bound to Hb, with only a small contribution from dissolved O2, in accordance with the O2 flux equation:
CO ([Hb] x SaO2 x 1.34 + paO2 x 0.003)
Thus, blood passing through high V/Q areas - with marginally increased O2 content - cannot compensate for blood passing through low V/Q areas with significantly lower O2 content